4. Gao F, Hou XZ, Liu YC. Study of the effect of IUGR during late pregnancy on the immune capability of neonatal lambs. Prog Nat Sci China (chinese) 2006; 16:1336ŌĆō40.
9. Greenwood PL, Bell AW. Consequences of intra-uterine growth retardation for postnatal growth, metabolism and pathophysiology. Reprod Suppl 2003; 61:195ŌĆō206.


10. Cromi A, Ghezzi F, Raffaelli R, Bergamini V, Siesto G, Bolis P. Ultrasonographic measurement of thymus size in IUGR fetuses: a marker of the fetal immunoendocrine response to malnutrition. Ultrasound Obstet Gynecol 2009; 33:421ŌĆō6.
https://doi.org/10.1002/uog.6320


11. Ekin A, Gezer C, Taner CE, Solmaz U, Gezer NS, Ozeren M. Prognostic value of fetal thymus size in intrauterine growth restriction. J Ultrasound Med 2016; 35:511ŌĆō7.
https://doi.org/10.7863/ultra.15.05039


12. Olearo E, Oberto M, Ogg├© G, et al. Thymic volume in healthy, small for gestational age and growth restricted fetuses. Prenat Diagn 2012; 32:662ŌĆō7.
https://doi.org/10.1002/pd.3883


13. Contreras YM, Yu X, Hale MA, et al. Intrauterine growth restriction alters T-lymphocyte cell number and dual specificity phosphatase 1 levels in the thymus of newborn and juvenile rats. Pediatr Res 2011; 70:123ŌĆō9.
http://doi.org/10.1203/PDR.0b013e31821f6e75


14. Liu Y, He S, Zhang Y, et al. Effects of intrauterine growth restriction during late pregnancy on the development of the ovine fetal thymus and the T-lymphocyte subpopulation. Am J Reprod Immunol 2015; 74:26ŌĆō37.
https://doi.org/10.1111/aji.12371


16. The State Science and Technology Commission of China. Regulations for administration of affairs concerning experimental animal. Beijing, China: The China Legal System Publishing House Press; 1988.
17. Symonds ME, Budge H, Stephenson T, McMillen IC. Fetal endocrinology and development-manipulation and adaptation to long-term nutritional and environmental challenges. Reproduction 2001; 121:853ŌĆō62.
https://doi.org/10.1530/rep.0.1210853


18. Gao F, Liu YC, Zhang C, Zhang ZH, Song SS. Effect of intrauterine growth restriction during late pregnancy on the growth performance, blood components, immunity and anti-oxidation capability of ovine fetus. Livest Sci 2013; 155:435ŌĆō41.
https://doi.org/10.1016/j.livsci.2013.04.016

19. Liu YC, Ma C, Li H, Li L, Gao F, Ao C. Effects of intrauterine growth restriction during late pregnancy on the cell apoptosis and related gene expression in ovine fetal liver. Theriogenology 2017; 90:204ŌĆō9.
https://doi.org/10.1016/j.theriogenology.2016.11.030


20. Sambrook J, Russell DW. Molecular cloning: a laboratory manual. 3nd edCold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press; 2001.
21. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(ŌłÆDelta Delta C(T)) method. Methods 2001; 25:402ŌĆō8.
https://doi.org/10.1006/meth.2001.1262


22. SAS Institute, Inc. SAS/STAT userŌĆÖs guide. Cary, NC, USA: SAS Institute, Inc; 2001.
24. Mitsumori K, Takegawa K, Shimo T, Onodera H, Yasuhara K, Takahashi M. Morphometric and immunohistochemical studies on atrophic changes in lympho-hematopoietic organs of rats treated with piperonyl butoxide or subjected to dietary restriction. Arch Toxicol 1996; 70:809ŌĆō14.
https://doi.org/10.1007/s002040050343


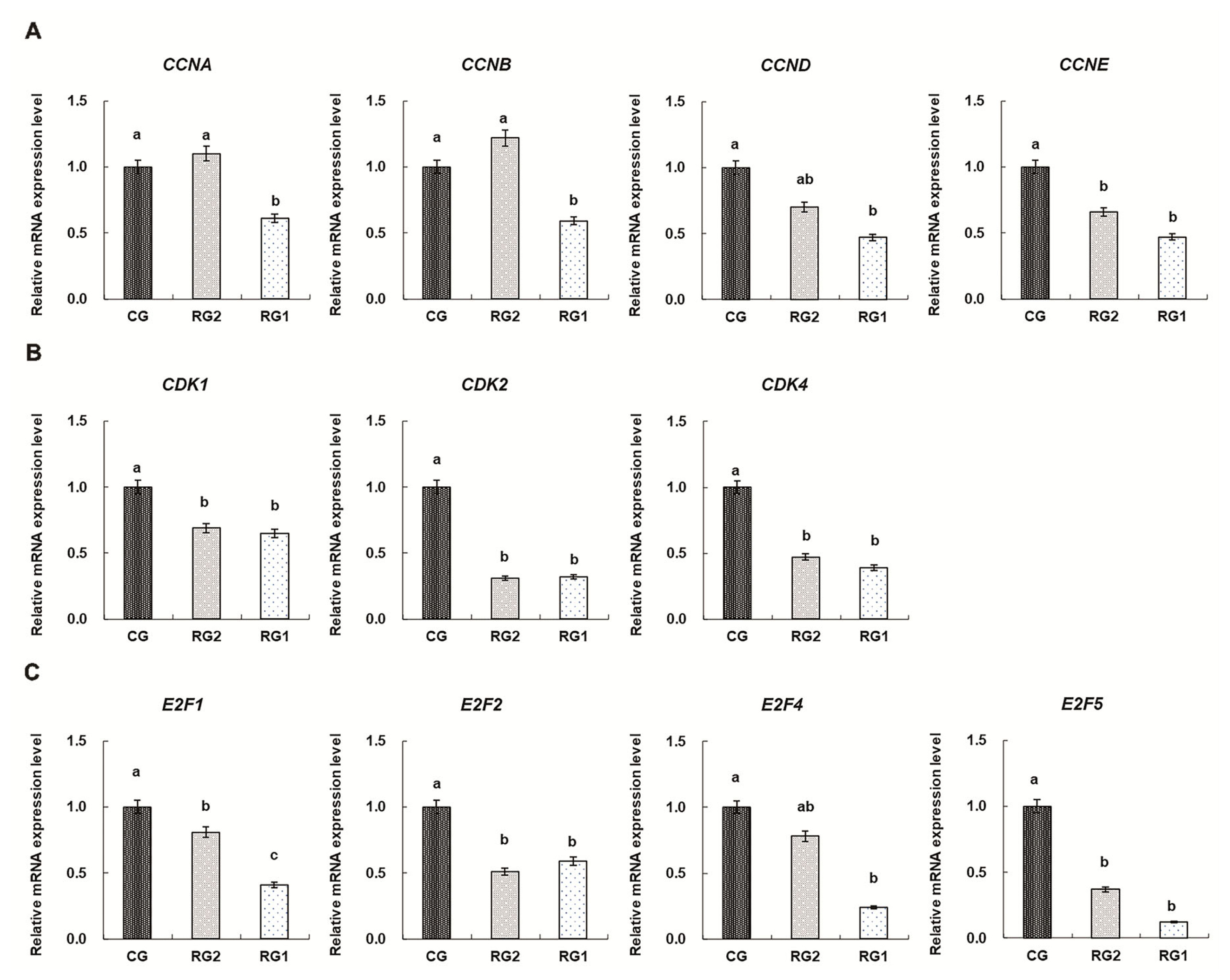
31. Satyanarayana A, Kaldis P. Mammalian cell-cycle regulation: several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene 2009; 28:2925ŌĆō39.
https://doi.org/10.1038/onc.2009.170


40. Boguszewski CL, Boguszewski MC, Kopchick JJ. Growth hormone, insulin-like growth factor system and carcinogenesis. Endokrynol Pol 2016; 67:414ŌĆō26.
https://doi.org/10.5603/EP.a2016.0053


42. Manley NR, Richie ER, Blackburn CC, Condie BG, Sage J. Structure and function of the thymic microenvironment. Front Biosci (Landmark Ed) 2011; 16:2461ŌĆō77.
https://doi.org/10.2741/3866


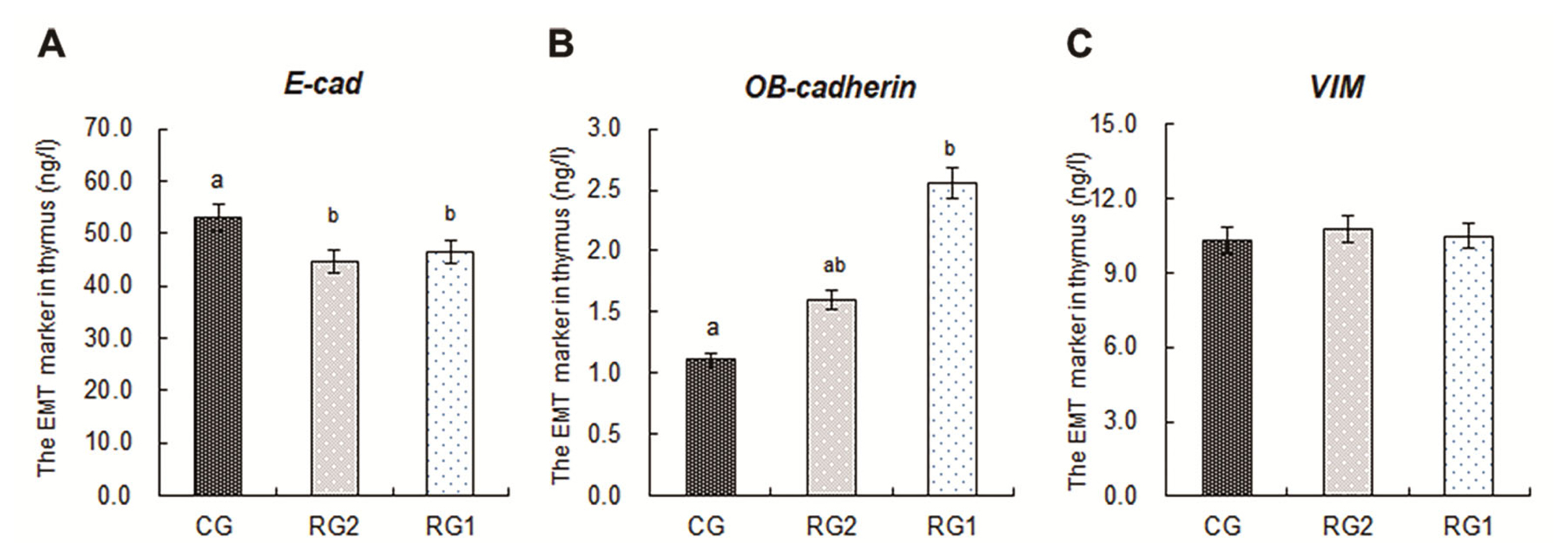
44. Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3ŌĆ▓ kinase/AKT pathways. Oncogene 2005; 24:7443ŌĆō54.
https://doi.org/10.1038/sj.onc.1209091











 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





