 |
 |
| Anim Biosci > Volume 36(2); 2023 > Article |
|
Abstract
Objective
This study evaluated the effects of dandelion supplements on lactation performance, circulating antioxidative activity and plasma metabolomics in primiparous dairy cows.
Methods
A total of 60 mid-lactation dairy cows (milk yield = 34.29±0.34 kg/d; days in milk = 151.72±2.36 days) were divided into 4 treatment groups randomly, comprising the addition of dandelion at 0, 100, 200, 400 g/d per head. The experiment lasted for 8 weeks with an extra 10 days’ pre-feeding period. Milk and blood samples were collected, and plasma samples were selected to perform metabolomics analysis.
Results
Supplementing 200 g/d of dandelion increased the yield of milk and lactose (p≤ 0.05). The milk somatic cell counts (p≤0.05) were lower in all dandelion groups than those in the control group. The activity of glutathione peroxidase (p≤0.05) and superoxide dismutase (p≤0.05) were increased and plasma malondialdehyde (p = 0.01) was decreased when cows were fed 200 g/d dandelion. Plasma metabolomics analysis showed that 23 hub differential metabolites were identified in the 200 g/d dandelion group. These metabolites such as ribose, glutamic acid, valine, and phenylalanine were enriched in D-glutamine and D-glutamate metabolism (p = 0.06, impact value = 1), phenylalanine, tyrosine, and tryptophan biosynthesis (p = 0.05, impact value = 0.5), and starch and sucrose metabolism (p = 0.21, impact value = 0.13). Moreover, correlation analysis showed that circulating ribose, mannose, and glutamic acid were positively related to milk yield.
Dandelion (Taraxacum mongolicum Hand.-Mazz.), a member of the Asteraceae family, contains various biological phytochemicals that are beneficial to animal health [1]. Generally, it is commonly used as a medicinal herb owing to its diuretic, antibacterial, anti-inflammatory, antidepressant, antiallergy, antioxidant, and choleretic properties. Previous studies have associated these properties with the presence of an array of secondary metabolites, including sesquiterpene lactones, phenolic compounds, polysaccharides, and flavonoids [2–4]. The use of medicinal herbs, as well as their associated secondary metabolites, in the livestock sector has attracted interest from animal researchers and practitioners [5], due to the existing challenges encountered during the application of these products in animal production [6].
Several studies have described the use of medicinal herbs in animal husbandry. The milk yield, rumen fermentation, and immune function were improved when dairy cows were fed with medicinal plants like triterpene saponins and Perilla frutescens leaf [7,8]. The addition of dandelion to the diet of weaning pigs was found to improve the growth rate, the efficiency of feed intake and energy digestibility [9]. Besides, dandelion extracts could enhance intestinal digestion and absorption in golden pompano [10]. However, the underlying mechanisms have not been explored sufficiently in these researches.
Metabolomics is a useful method to explore the essential and complex changes in various biological systems. In our previous study, the rumen fluid metabolome was found to improve the tryptophan metabolism, starch and sucrose metabolism, and pentose phosphate pathway [11]. However, information about the effects of dandelion on lactation parameters and plasma metabolomics in dairy cows is relatively limited. Hence, we sought to evaluate the effect of dandelion supplementation on lactation performance, plasma variables and metabolome in dairy cows.
The experimental procedures were approved by the Animal Care Committee of Zhejiang University and complied with the guidelines of animal research (Protocol No. ZJU20160379, Hangzhou, China).
A total of 60 mid-lactating primiparous dairy cows were selected and blocked based on milk yield and days in milk (milk yield = 34.29±0.34 kg/d; days in milk = 151.72±2.36 days), then randomly assigned to 4 dietary groups (n = 15/treatment). The cows were maintained on the same basal diet supplemented with 0, 100, 200, or 400 g/d of dandelion per head. We supplemented the dandelion individually before the total mixed ration (TMR) was fed and confirmed that the dairy cows had eaten all the dandelion. The dandelion plants, comprising whole-crop in an air-dried form, including stalks, leaves, and flowers, were planted, and harvested by the dairy farm of Yinxiang Weiye Group. Dandelion was ground, sieved through a 1-cm screen, then stored in sealed bags until feeding. The nutrient composition of basal TMR and the bioactive compounds of dandelion are outlined in supplemental Table 1 and supplemental Table 2. Cows were housed in a sand-bedded free-stall barn, with feed available ad libitum and free access to water. Milking and feeding procedures were performed 3 times a day (at 07:00, 14:30, and 21:30), with an 8 week formal feeding and a 10 days’ pre-feeding period.
Dry matter intake was calculated daily by the TMR and refusals of each group. During the trial, TMR samples were collected on the second and third day of each week, then mixed to make a composite sample for each week and treatment. Samples of individual concentrates, as well as forages, were also collected weekly and immediately stored at −20°C. Part of the samples were dried in a forced-air oven, at 65°C for 48 h, then used for the determination of dry matter (DM). Others were ground in a Wiley Mill (1-mm screen; Thomas Scientific, Swedesboro, NJ, USA) and used for compositional analyses. Composite samples of forages and concentrates were analyzed for DM, crude protein, and ether extract [12]. The content of acid detergent fiber and neutral detergent fiber were determined by an Ankom A200 apparatus (Ankom Technology, Macedon, NY, USA) with heat-stable amylase and sodium sulfite (Fisher Scientific, Waltham, MA, USA) and expressed inclusive of residual ash [13].
Milk samples were collected on the sixth day of each week, 50 mL of milk sample was collected from each cow, and samples across the 3 milking times per day at a ratio of 4:3:3 as earlier described [14]. The 50 mL composite milk samples were stored in a sample bottle, with the addition of 0.6 mg/mL potassium dichromate to act as a preservative, then stored at 4°C to await further analysis. Thereafter, an automatic ultrasonic milk composition analyzer (Bentley Instruments, Chaska, MN, USA) was used to determine milk components, including protein, fat, lactose contents, as well as somatic cell count (SCC), and milk urea nitrogen.
Blood samples (10 mL) were collected from the coccygeal vein into evacuated tubes containing sodium heparin before morning feeding on the fifth day of week 1, 4, and 8. Plasma was then obtained from the sampled blood, by centrifugation at 3,000×g for 10 min at 4°C and stored at −20°C for further analysis. Glucose, nonesterified fatty acids, blood urea nitrogen, total protein, bilirubin, albumin, triglyceride, globulin, β-hydroxybutyrate, creatinine, aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase concentrations in plasma samples were analyzed by an auto-analyzer 7020 (Hitachi High-Technologies Corp., Tokyo, Japan) according to the method described in previous study [15]. Commercial assay kits of Jiangsu Nanjing Biological Technology Co. Ltd., Jiangsu, China, were then used to determine the enzymatic activity of malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and total antioxidant capacity as previously described [16].
Sixteen plasma samples (8 in control group, 8 in 200 g/d dandelion group) were selected to perform the metabolomics analysis according to the phenotype of milk yield, feed intake, and milk quality. Fifty μL sample was transferred into a 1.5 mL tube, then added 200 μL of pre-chilled methanol containing 5 μL internal standard (adenosine, 0.5 mg/mL stock solution) and vortexed for 30 s. Samples were sonicated in ice water for 10 minutes. After centrifugation at 4°C and 10,000 rpm for 15 minutes, 180 μL of the supernatant was transferred to a fresh tube. To prepare quality control (QC) sample, 30 μL of each sample was taken out and combined. After evaporation in a vacuum concentrator, 30 μL of Methoxyamination hydrochloride (20 mg/mL in pyridine) was added and then incubated at 80°C for 30 minutes, then derivatized by 40 μL Bis(trimethylsilyl)trifluoroacetamide (BSTFA) reagent (1% Trimethylchlorosilane, v/v) for 1.5 hours at 70°C. Gradually cooling samples to room temperature and added 5 μL fatty acid methyl esters (in chloroform) to the QC sample. All samples were analyzed by gas chromatography combined with a time-of-flight mass spectrometer (GC-TOF-MS).
GC-TOF-MS analysis was performed using an Agilent 7890 gas chromatograph coupled with a time-of-flight mass spectrometer. The system utilized a DB-5MS capillary column. One μL aliquot sample was injected in a splitless manner. Helium was used as the carrier gas, the front inlet purge flow was 3 mL/min, and the gas flow rate through the column was 1 mL/min. The initial temperature was kept at 50°C for 1 minute, then increased to 310°C at a rate of 20°C/min, held at 310°C for 8 minutes. The temperature of the injection line, transfer line, and ion source was 280°C, 280°C, and 250°C, respectively. In the electron impact mode, the energy was −70 eV. After a solvent delay of 4.8 minutes, mass spectrometry data were acquired in the full scan mode with the range of 50 to 500 m/z.
Raw data analysis, including peak extraction, baseline ad justment, deconvolution, comparison, and integration, was finished with Chroma TOF (V 4.3×, LECO) software and the LECO-Fiehn Rtx5 database was used for metabolite identification by matching the mass spectrum and retention index. Finally, less than half of the peaks detected in QC samples or relative standard deviation >30% in QC samples was removed.
All data were analyzed by the mixed model (SAS 9.2, 2000) and checked for normality by the UNIVARIATE procedure. A randomized block design, with repeated measures, was used for the analyses, with the treatment and week as the fixed effects, the cow as a random effect. Contrasts were constructed to examine treatment effects and dandelion supplementation levels, with orthogonal polynomials accounting for the unequal spacing of dandelion levels. The differences between the groups were evaluated by Tukey’s multiple range test. Data are expressed as least squares means and standard errors of the mean. Correlation analyses between lactation parameters and plasma metabolites were calculated using spearman’s correlation test. All analyses were performed at p≤0.05 for determination of statistical significance, with 0.05<p≤0.10 indicated trends.
A quadratic increase in milk yield (p = 0.04) was observed, with the highest level recorded in the group fed on 200 g/d dandelion (Table 1). In addition, the energy corrected milk (ECM) (p = 0.03) and lactose (p = 0.05) yield were higher in cows fed on 200 g/d dandelion relative to those in the control group. The SCC (p = 0.01) was lower in all dandelion addition groups. The yield of milk was higher in the whole experiment period, while the results showed significant in the first 5 weeks when cows were fed on 200 g/d of dandelion (Figure 1).
The concentration of glucose was higher in the 200 and 400 g/d groups (p = 0.05), compared with the 0 and 100 g/d groups (Table 2). Plasma SOD activity was increased, and MDA concentration was decreased in the group supplemented with 400 g/d dandelion (p = 0.05). Moreover, dietary 200 g/d dandelion supplementation significantly increased plasma activity of GSH-Px (p = 0.05), and SOD (p = 0.05), decreased the concentration of MDA (p = 0.01).
According to the results of milk performance in dairy cows, optimal supplementation dosage of dandelion was confirmed, and 16 plasma samples were selected from the 0 (CON) and 200 g/d (DAN) groups. The analysis of orthogonal partial least squares-discriminant analysis (OPLS-DA) and principal component analysis of the metabolic profiles showed a separate trend between the CON and DAN groups (Figure 2), suggesting that the model of plasma metabolomics could be used to identify the difference between the two groups.
In general, 23 differential metabolites were identified in the CON and DAN groups (p<0.05, VIP>1), with 19 metabolites upgraded and 4 downgraded (Table 3), including amino acids (valine, cystine, glutamic acid, phenylalanine, and threonine), organic acids (palmitic acid, methylmalonic acid, and salicylic acid), and sugars (mannose, glucose, glucose-1-phosphate, and ribose).
The plasma metabolome view map of significant metabolic pathways characterized in the CON and DAN group is shown in Figure 3. Enrichment and pathway topology analysis showed that 6 pathways exhibited an important value at the meaningful level (impact value >0.10): Phenylalanine, tyrosine and tryptophan biosynthesis (p = 0.05, impact value = 0.5), D-glutamine and D-glutamate metabolism (p = 0.06, impact value = 1), Starch and sucrose metabolism (p = 0.21, impact value = 0.13), Phenylalanine metabolism (p = 0.15, impact value = 0.36), Alanine, aspartate and glutamate metabolism (p = 0.31, impact value = 0.20), Arginine biosynthesis (p = 0.17, impact value = 0.12).
Correlation analyses between the lactation performance and plasma differential metabolites is shown in Figure 4. Ribose, mannose, and glutamic acid were positively correlated with milk yield (p<0.05). Mannose was positively correlated with lactose (p<0.05). Ribose, mannose, glucose-1-phosphate were negatively correlated with fat (p<0.05). Most of differential metabolites were negatively correlated with SCC (p<0.05).
Lactation performance is an important and comprehensive parameter for dairy husbandry. In previous study, we have identified the bioactive compounds in dandelion, among them, the most abundant compound is β-D-glucopyranoside, which functioned to stimulate the process of digestion. Besides, the rumen microorganisms that correlated with the degrading of cellulose and structure carbohydrate were enriched when the dandelion was supplemented [11]. Another study has reported an increase in growth performance, the efficiency of feed intake, and energy digestibility in animals fed on dandelion [9]. Functional foods like dandelion can enhance digestibility, owing to the presence of bitter sesquiterpene lactones [17]. Hence, the increase in ECM and lactose yield in dandelion-fed dairy cows may be attributed to the higher milk yield and digestion-enhancing activities. These results indicated that the lactation performance could be improved with the supplementation of dandelion on mid-lactation dairy cows.
Notably, supplementing feed with dandelion led to a sig nificantly lower SCC in dairy cows in the treated group relative to controls, suggesting an important role of dandelion in improving milk quality. Dandelion flowers are a potential source of natural antioxidants due to the higher content of phenolic compounds [1]. The effects of the crude extract of flowers in inhibiting the damage caused by reactive oxygen species and the damage caused by nitric oxide were attributed to caffeic acid, luteolin 7-glucoside, flavonoid luteolin, and chlorogenic acid [18–19]. Moreover, dietary dandelion supplementation significantly increased plasma concentration of GSH-Px and SOD, decreased the MDA. It is generally accepted that SOD plays a significant role in the conversion of oxygen radicals to peroxides [20], whereas MDA is an indicator of oxidative stress owing to its activity as a lipid peroxidation product [21]. Previous studies have shown that blood MDA and SOD are negatively correlated [22]. In this study, the low MDA and high SOD concentrations in plasma obtained from cows in the dandelion group might indicate that this plant reduces oxidative stress in cows. Besides, 2-Hydroxypyridine was negatively correlated with SCC, which could combine with copper to function as the most potent SOD mimic [23]. Therefore, supplementing feed with dandelion might improve the antioxidant activities of dairy cows, which could then enhance the health status and lactating performance.
Metabolomics analysis revealed that the levels of valine, phenylalanine and glutamate acid were enriched in the DAN group, suggesting that an increasing trend of milk protein synthesis. A previous study has shown that feeding rumen-degradable valine could increase the milk yield in late-lactating dairy cows [24]. Besides, in MAC-T cells, valine and phenylalanine could stimulate milk protein synthetic and energy-mediated pathways differentially [25]. The results showed that phenylalanine and metabolism phenylalanine, tyrosine, and tryptophan biosynthesis were mainly affected by phenylalanine and tyrosine. Phenylalanine could also be able to convert into Tyr in the mammary gland of ruminant animals and regulate the synthesis or secretion of lipase, trypsin, and α-amylase through mRNA translation initiation factors ribosomal protein S6 kinase 1 (S6K1) and 4E-binding protein 1 (4EBP1) in dairy calves [26,27]. These findings indicated that the significantly different metabolites could play an important role in the milk synthesis.
For the metabolic pathways, phenylalanine, tyrosine and tryptophan biosynthesis, D-glutamine and D-glutamate, starch and sucrose metabolism were identified in this study. D-glutamine and D-glutamate metabolism were affected by glutamate, which could be transferred from glutamic acid. As an NEAA, previous studies had shown that glutamate could limit the synthesis of protein [28] and decrease the SCC of milk and improve the health status of high-yielding dairy cows [29]. Correlation analysis results also revealed that the ribose, mannose, and glutamic acid were positively correlated with milk yield. Thus, it is possible to infer that the improved amino acid and carbohydrate metabolism could provide adequate nutrients for the lactation activity of dairy cows. Further studies should aim to extract the specific composition and investigate the underlying mechanisms in the milk synthesis.
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
FUNDING
This work was financially supported by China Agriculture Research System (No. CARS-36). The authors appreciate the staff of Yinxiang Weiye Dairy Farm (Shandong, China) for their assistance in feeding, milking, and caring for the animals. We also acknowledge the members of the Institute of Dairy Science Zhejiang University for their assistance with blood sampling.
Figure 1
Changes in milk yield of mid-lactating cows fed on a basal diet with 0, 100, 200, 400 g/d of dandelion supplemented. Bars indicate standard error of the mean; Asterisk (*) indicates significant differences (p<0.05) between the control (0) and dandelion (200 g/d) groups.
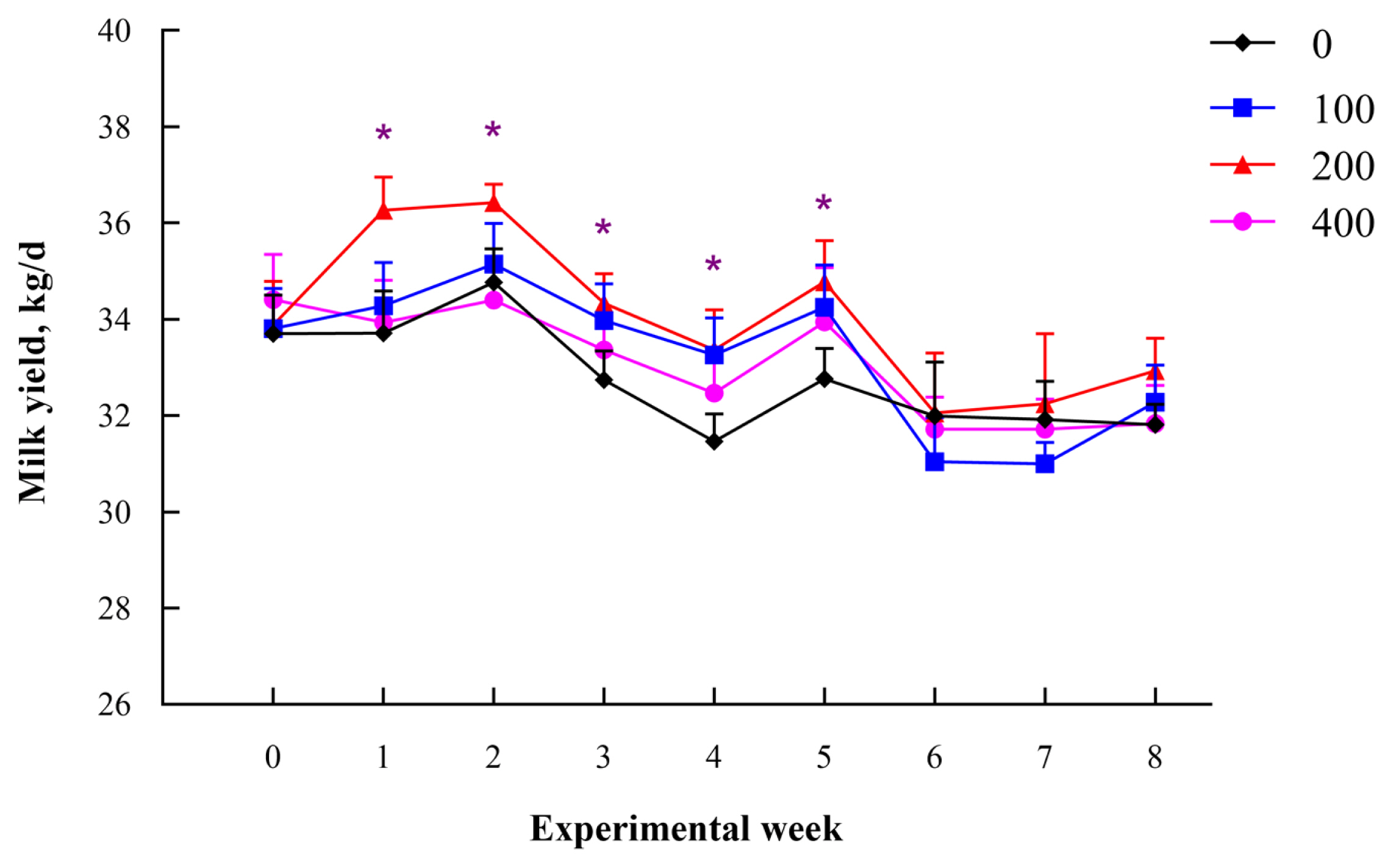
Figure 2
The multivariate statistical analysis of CON and DAN plasma metabolome. The red circle represents DAN, while the blue box represents CON. This figure contains the PCA score map (a) OPLS-DA score plot (b) and permutation plot (c) derived from the metabolite profiles of dairy cows. PCA, principal component analysis; OPLS-DA, orthogonal partial least squares discriminant analysis. CON, control group; DAN, 200 g/d of dandelion supplemented group.
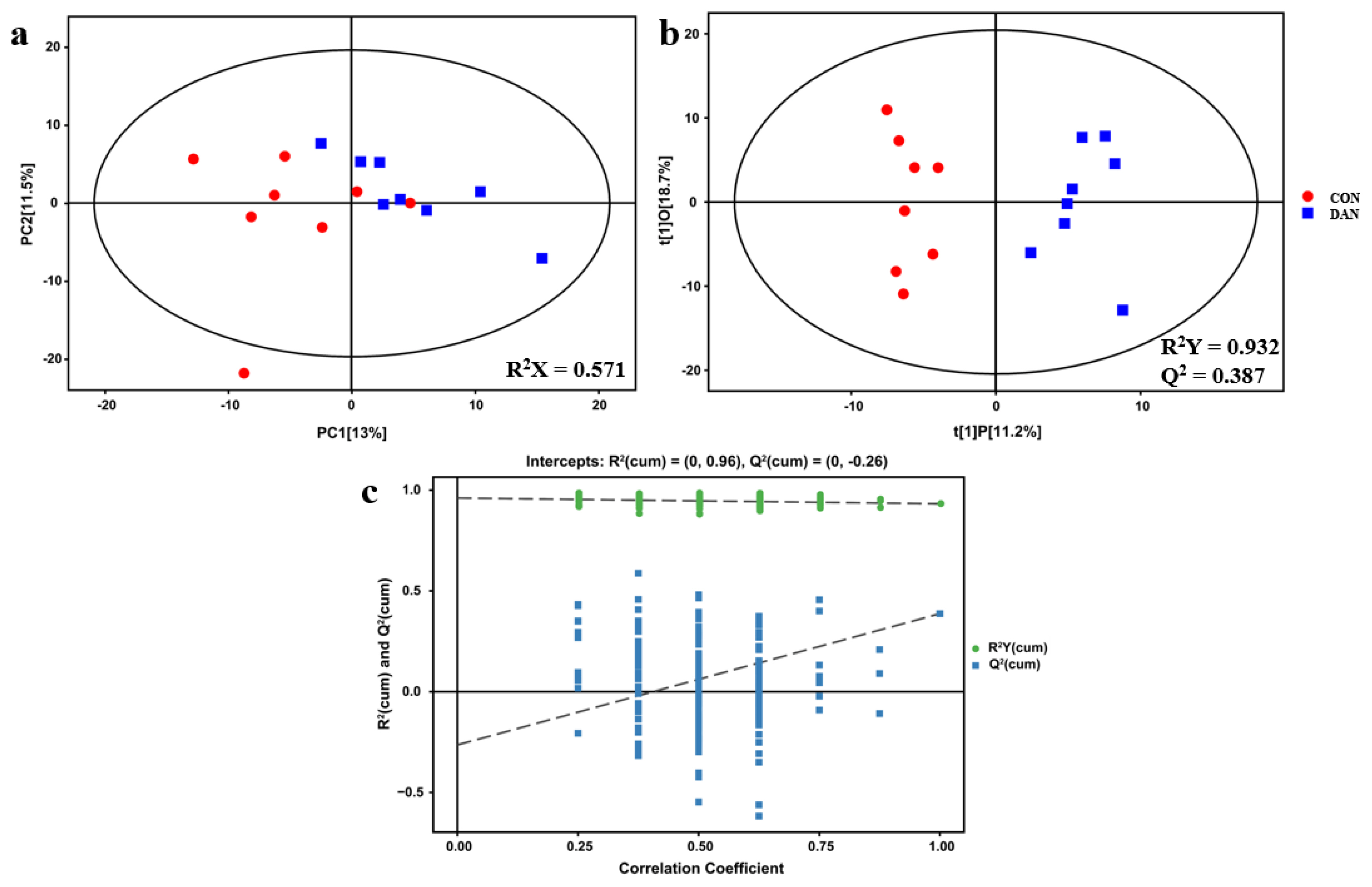
Figure 3
The metabolome view map of significant metabolic pathways characterized in plasma for cows in the CON and DAN group. The x-axis represents pathway enrichment, and the y-axis represents pathway impact. Larger sizes and higher impact values, respectively. CON, control group; DAN, 200 g/d of dandelion supplemented group.

Figure 4
Correlation analyses between the lactation performance and plasma differential metabolites. The red squares represent the positive correlations while the blue ones represent the negative correlations. * p<0.05. MUN, milk urea nitrogen; SCC, somatic cell counts.

Table 1
Effects of dietary dandelion supplements on feed intake and lactation performance in primiparous dairy cows
| Item | Dandelion (g/d) | SEM | p-value1) | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| 0 | 100 | 200 | 400 | T | L | Q | ||
| DMI (kg/d) | 20.6 | 20.7 | 20.8 | 20.6 | - | - | - | - |
| Milk yield (kg/d) | ||||||||
| Raw | 32.9b | 33.9ab | 34.7a | 33.3b | 0.26 | 0.03 | 0.63 | 0.04 |
| ECM2) | 32.6b | 33.6ab | 34.8a | 33.2b | 0.35 | 0.04 | 0.83 | 0.03 |
| Fat | 1.27 | 1.32 | 1.37 | 1.30 | 0.032 | 0.45 | 0.78 | 0.37 |
| Protein | 1.05 | 1.08 | 1.12 | 1.07 | 0.021 | 0.39 | 0.86 | 0.48 |
| Lactose | 1.66b | 1.70b | 1.75a | 1.68b | 0.034 | 0.05 | 0.75 | 0.05 |
| Content (g/100 g) | ||||||||
| Fat | 3.87 | 3.89 | 3.94 | 3.90 | 0.024 | 0.48 | 0.78 | 0.81 |
| Protein | 3.27 | 3.25 | 3.29 | 3.27 | 0.075 | 0.62 | 0.29 | 0.56 |
| Lactose | 5.14 | 5.12 | 5.15 | 5.12 | 0.047 | 0.49 | 0.87 | 0.50 |
| MUN (mg/dL) | 11.4 | 10.8 | 11.1 | 12.0 | 0.39 | 0.16 | 0.55 | 0.30 |
| SCC (103/mL) | 199.2a | 159.7b | 147.3b | 105.0b | 0.10 | <0.01 | 0.01 | 0.51 |
Table 2
Effects of dietary dandelion supplements on plasma variables in primiparous dairy cows
| Item | Dandelion (g/d) | SEM | p-value1) | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| 0 | 100 | 200 | 400 | T | L | Q | ||
| Total protein (g/L) | 73.9 | 73.5 | 74.8 | 72.2 | 0.59 | 0.54 | 0.79 | 0.37 |
| Albumin (g/L) | 34.4 | 34.5 | 33.7 | 34.0 | 0.27 | 0.70 | 0.71 | 0.47 |
| Globulin (g/L) | 39.4 | 39.0 | 41.0 | 38.2 | 0.76 | 0.63 | 0.86 | 0.34 |
| Bilirubin (μmol/L) | 3.60 | 3.78 | 3.76 | 3.96 | 0.082 | 0.49 | 0.89 | 0.48 |
| Glucose (mmol/L) | 3.45b | 3.50b | 3.60a | 3.56a | 0.023 | 0.22 | 0.83 | 0.05 |
| Creatinine (μmol/L) | 60.3 | 61.6 | 63.2 | 63.7 | 0.95 | 0.58 | 0.94 | 0.59 |
| Triglyceride (mmol/L) | 0.15 | 0.16 | 0.17 | 0.15 | 0.012 | 0.20 | 0.47 | 0.33 |
| ALT (U/L) | 29.0 | 28.6 | 26.5 | 28.3 | 0.54 | 0.39 | 0.86 | 0.57 |
| AST (U/L) | 87.6 | 89.4 | 96.3 | 87.3 | 1.88 | 0.96 | 0.29 | 0.48 |
| ALP (U/L) | 48.5 | 46.1 | 47.4 | 39.6 | 1.56 | 0.44 | 0.59 | 0.37 |
| BUN (mmol/L) | 4.88 | 4.74 | 5.31 | 4.96 | 0.123 | 0.28 | 0.47 | 0.39 |
| NEFA (μmol/L) | 137.2 | 155.6 | 145.3 | 155.0 | 5.34 | 0.59 | 0.86 | 0.39 |
| MDA (μmol/L) | 3.93a | 2.95b | 2.13b | 2.04b | 0.020 | 0.05 | 0.01 | 0.21 |
| SOD (U/mL) | 163.5b | 165.1b | 168.6a | 169.9a | 1.83 | 0.04 | 0.05 | 0.85 |
| GSH-Px (U/L) | 216.9b | 228.4b | 249.2a | 222.8b | 5.04 | 0.03 | 0.47 | 0.05 |
| T-AOC (mmol/L) | 0.52 | 0.53 | 0.52 | 0.52 | 0.011 | 0.93 | 0.93 | 0.58 |
Table 3
| Metabolites | Similarity | VIP | FC2) | p-value |
|---|---|---|---|---|
| Dodecanol | 253 | 1.75 | 2.63 | <0.01 |
| 9-Fluorenone | 385 | 1.52 | 2.05 | 0.01 |
| Glucose | 850 | 2.26 | 2.00 | <0.01 |
| Ribose | 713 | 1.23 | 1.94 | 0.03 |
| Valine | 905 | 1.58 | 1.81 | 0.03 |
| Salicylic acid | 451 | 1.37 | 1.68 | 0.02 |
| Thioctamide | 257 | 1.60 | 1.63 | 0.04 |
| Glutamic acid | 690 | 1.14 | 1.54 | 0.02 |
| Phenylalanine | 836 | 1.06 | 1.47 | 0.03 |
| 2-Amino-3-phosphonopropanoic acid | 292 | 2.15 | 1.34 | 0.01 |
| Dihydrocarveol | 313 | 2.13 | 1.31 | 0.01 |
| Cytidine-monophosphate | 466 | 1.57 | 1.26 | 0.04 |
| 1-Methylhydantoin | 202 | 1.84 | 1.22 | 0.04 |
| Palmitic acid | 935 | 1.49 | 1.21 | 0.04 |
| Glucose-1-phosphate | 777 | 1.72 | 1.17 | 0.05 |
| Mannose | 888 | 1.60 | 1.16 | 0.03 |
| 2-Hydroxypyridine | 887 | 1.79 | 1.15 | 0.03 |
| Cystine | 232 | 1.64 | 1.14 | 0.04 |
| Methylmalonic acid | 572 | 1.48 | 1.10 | 0.04 |
| Maleamate | 452 | 1.69 | 0.59 | 0.03 |
| Digitoxose | 249 | 1.87 | 0.52 | 0.05 |
| Threonine | 539 | 1.92 | 0.43 | 0.03 |
| Indolelactate | 533 | 1.82 | 0.39 | 0.03 |
REFERENCES
1. González-Castejón M, Visioli F, Rodriguez-Casado A. Diverse biological activities of dandelion. Nutr Rev 2012; 70:534–47.
https://doi.org/10.1111/j.1753-4887.2012.00509.x


2. Cho SY, Park JY, Park EM, et al. Alternation of hepatic antioxidant enzyme activities and lipid profile in streptozotocin-induced diabetic rats by supplementation of dandelion water extract. Clin Chim Acta 2002; 317:109–17.
https://doi.org/10.1016/s0009-8981(01)00762-8


3. Schütz K, Carle R, Schieber A. Taraxacum--a review on its phytochemical and pharmacological profile. J Ethnopharmacol 2006; 107:313–23.
https://doi.org/10.1016/j.jep.2006.07.021


4. Choi UK, Lee OH, Yim JH, et al. Hypolipidemic and antioxidant effects of dandelion (Taraxacum officinale) root and leaf on cholesterol-fed rabbits. Int J Mol Sci 2010; 11:67–78.
https://doi.org/10.3390/ijms11010067



5. Mertenat D, Cero MD, Vogl CR, et al. Ethnoveterinary knowledge of farmers in bilingual regions of Switzerland - is there potential to extend veterinary options to reduce antimicrobial use? J Ethnopharmacol 2020; 246:112184
https://doi.org/10.1016/j.jep.2019.112184



6. Hristov AN, Oh J, Firkins JL, et al. Special topics--Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options. J Anim Sci 2013; 91:5045–69.
https://doi.org/10.2527/jas.2013-6583


7. Wang B, Ma MP, Diao QY, Tu Y. Saponin-induced shifts in the rumen microbiome and metabolome of young cattle. Front Microbiol 2019; 10:356
https://doi.org/10.3389/fmicb.2019.00356



8. Sun Z, Yu Z, Wang B. Perilla frutescens leaf alters the rumen microbial community of lactating dairy cows. Microorganisms 2019; 7:562
https://doi.org/10.3390/microorganisms7110562



9. Yan L, Zhang ZF, Park JC, Kim IH. Evaluation of houttuynia cordata and taraxacum officinale on growth performance, nutrient digestibility, blood characteristics, and fecal microbial shedding in diet for weaning pigs. Asian-Australas J Anim Sci 2012; 25:1439–44.
https://doi.org/10.5713/ajas.2012.12215



10. Tan X, Sun Z, Zhou C, et al. Effects of dietary dandelion extract on intestinal morphology, antioxidant status, immune function and physical barrier function of juvenile golden pompano Trachinotus ovatus. Fish Shellfish Immunol 2018; 73:197–206.
https://doi.org/10.1016/j.fsi.2017.12.020


11. Li Y, Lv M, Wang J, et al. Dandelion (Taraxacum mongolicum Hand.-Mazz.) supplementation-enhanced rumen fermentation through the interaction between ruminal microbiome and metabolome. Microorganisms 2020; 9:83
https://doi.org/10.3390/microorganisms9010083



12. AOAC. Official methods of analysis. I15th edArlington, VA, USA: Association of Official Analytical Chemists; 1990.
13. Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 1991; 74:3583–97.
https://doi.org/10.3168/jds.S0022-0302(91)78551-2


14. Wang B, Mao SY, Yang HJ, et al. Effects of alfalfa and cereal straw as a forage source on nutrient digestibility and lactation performance in lactating dairy cows. J Dairy Sci 2014; 97:7706–15.
https://doi.org/10.3168/jds.2014-7961


15. Wang DM, Chacher B, Liu HY, Wang JK, Lin J, Liu JX. Effects of γ-aminobutyric acid on feed intake, growth performance and expression of related genes in growing lambs. Animal 2015; 9:445–8.
https://doi.org/10.1017/S1751731114002651


16. Zhao H, Lan Y, Liu H, et al. Antioxidant and hepatoprotective activities of polysaccharides from spent mushroom substrates (Laetiporus sulphureus) in acute alcohol-induced mice. Oxid Med Cell Longev 2017; 2017:5863523
https://doi.org/10.1155/2017/5863523



17. Valussi M. Functional foods with digestion-enhancing properties. Int J Food Sci Nutr 2012; 63:Suppl 182–9.
https://doi.org/10.3109/09637486.2011.627841


18. Kitts DD, Wijewickreme AN. Effect of dietary caffeic and chlorogenic acids on in vivo xenobiotic enzyme systems. Plant Foods Hum Nutr 1994; 45:287–98.
https://doi.org/10.1007/BF01094097


19. Hu C, Kitts DD. Luteolin and luteolin-7-O-glucoside from dandelion flower suppress iNOS and COX-2 in RAW264.7 cells. Mol Cell Biochem 2004; 265:107–13.
https://doi.org/10.1023/b:mcbi.0000044364.73144.fe


20. Bernabucci U, Ronchi B, Lacetera N, Nardone A. Markers of oxidative status in plasma and erythrocytes of transition dairy cows during hot season. J Dairy Sci 2002; 85:2173–9.
https://doi.org/10.3168/jds.S0022-0302(02)74296-3


21. Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 1990; 186:421–31.
https://doi.org/10.1016/0076-6879(90)86135-i


22. Wang Y, Walsh SW. Increased superoxide generation is associated with decreased superoxide dismutase activity and mRNA expression in placental trophoblast cells in pre-eclampsia. Placenta 2001; 22:206–12.
https://doi.org/10.1053/plac.2000.0608


23. Suksrichavalit T, Prachayasittikul S, Nantasenamat C, Isarankura-Na-Ayudhya C, Prachayasittikul V. Copper complexes of pyridine derivatives with superoxide scavenging and antimicrobial activities. Eur J Med Chem 2009; 44:3259–65.
https://doi.org/10.1016/j.ejmech.2009.03.033


24. Hultquist KM, Casper DP. Effects of feeding rumen-degradable valine on milk production in late-lactating dairy cows. J Dairy Sci 2016; 99:1201–15.
https://doi.org/10.3168/jds.2015-10197


25. Kim J, Lee JE, Lee JS, Park JS, Moon JO, Lee HG. Phenylalanine and valine differentially stimulate milk protein synthetic and energy-mediated pathway in immortalized bovine mammary epithelial cells. J Anim Sci Technol 2020; 62:263–75.
https://doi.org/10.5187/jast.2020.62.2.263



26. Wang F, Shi H, Wang S, Wang Y, Cao Z, Li S. Amino acid metabolism in dairy cows and their regulation in milk synthesis. Current drug metabolism 2019; 20:36–45.
https://doi.org/10.2174/1389200219666180611084014


27. Guo L, Tian H, Shen J, et al. Phenylalanine regulates initiation of digestive enzyme mRNA translation in pancreatic acinar cells and tissue segments in dairy calves. Biosci Rep 2018; 38:BSR20171189
https://doi.org/10.1042/BSR20171189



28. Meijer GA, van der Meulen J, van Vuuren AM. Glutamine is a potentially limiting amino acid for milk production in dairy cows: a hypothesis. Metabolism 1993; 42:358–64.
https://doi.org/10.1016/0026-0495(93)90087-5








 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement
Supplement Print
Print





