 |
 |
| Anim Biosci > Volume 36(1); 2023 > Article |
|
Abstract
Objective
Current quail production is configured as an economic activity in scale. Advancements in quail nutrition have been limited to areas such as breeding and, automation of facilities and ambience. The objective of this study was to evaluate the performance responses, liver and oviduct morphometry, and liver histology of Japanese laying quails subjected to different levels of nitrogen-corrected apparent metabolizable energy (MEn).
Methods
A completely random design was used that consisted of nine levels of MEn, six replicates, and five hens per cage with a total of 270 quails. The experimental period lasted for 10 weeks. The variables of performance were subjected to analysis of variance and then regression analysis using the broken-line model. The morphometric and histological variables were subjected to multivariate exploratory techniques.
Results
The MEn levels influenced the responses to zootechnical performance. The broken-line model estimated the maximum responses for feed intake, egg production, egg weight, and egg mass as 3,040, 2,820, 1,802, and 2,960 kcal of MEn per kg of diet, respectively. Multivariate analysis revealed that the occurrence of hepatic steatosis and increased levels of Kupffer cells were not related to MEn levels.
Advances in genetics have allowed for the segregation of the multiplication and production sectors, and this is indicative of the degree of professionalization that the quail egg industry has achieved in recent decades. The facilities were adapted for Japanese quails, and currently, sheds possessing temperature control automation, dung removal, and egg collection are present at the main commercial egg-producing locations [1–3].
Attention should be focused on the concentration of nitrogen-corrected apparent metabolizable energy (MEn) in the diet. Therefore, the concentration of MEn in the diet should be established based on certain criteria. However, publications during the last two decades do not show a consensus regarding the amount of MEn required. Here, it is understood that a reduction in the difference between the values recommended in the literature is necessary. We located 11 studies examining MEn for quail hens, and the recommendations ranged from 2,600 to 3,100 kcal/kg and egg production (EP) ranged from 77% to 94% using Japanese and European quails [4–14]. When analyzing these publications, a common theme was that the mean concentration of MEn in the diet was 2,876 kcal/kg with an amplitude ranging from 87% to 110%. The lowest MEn tested was 2,500 kcal/kg [11]. The association of narrow amplitude with the ad libitum feed intake (FI) option may have been an attenuating factor in the imposed deficiency and, consequently, in the absence of an effect of the MEn levels tested in most of the studies reviewed here.
The possibility of obtaining a greater amplitude of the MEn values in the experimental diets may involve the use of the dilution technique; however, this is a common practice in amino acid studies [15]. For MEn, only one study using commercial laying hens utilized this technique [15], and with quails, the use of this technique is nonexistent. Another missing description in the literature is the impact of the concentration of MEn on hepatic physiology and oviduct morphometry of current Japanese quail lines improved for commercial posture. For other nutrients such as protein, the possible effects have already been described [16]. Thus, in this study we investigated the performance responses, liver and oviduct morphometry, and hepatic histology of laying Japanese quails subjected to different levels of MEn.
The Animal Ethics and Welfare Committee of Universidade Estadual Paulista approved all experimental procedures used in this study (protocol number 6.725/15).
Two hundred and seventy female Japanese quail (VICAMI strain at 16 weeks of age) were housed in galvanized wire cages measuring 1.0 m×0.5 m×0.15 m. The cages were equipped with feeders and nipple drinkers in a climatic chamber at room temperature. The birds were selected and uniformly distributed in the experimental units based on their weight (185±7 g) and EP (78%±6%). The light program that was used was a 16 L:8 D. The minimum, average, and maximum temperatures were 18°C, 22°C, and 26°C, respectively. The minimum, average, and maximum humidity values were 50%, 60%, and 68%, respectively. The experiment lasted ten weeks. The first four weeks involved adaptation, and the last six weeks were for data collection. All birds were fed 27 g of the diet per day and were supplied twice each day (morning and afternoon).
The treatments were distributed in a completely randomized design that consisted of nine increasing levels of MEn (1,609, 1,740, 1,895, 2,260, 2,394, 2,643, 2,892, 3,045, and 3,058 kcal/kg, respectively) with six replicates of five quails per experimental unit. Two diets were formulated with high and low MEn contents (Table 1) according to previously published recommendations [17] (Table 2).
The high MEn diet was formulated to contain 114% greater than the MEn recommendation (2,850 kcal/kg), and the low MEn diet was formulated to contain approximately 52% less than the MEn recommendation. The remaining samples were obtained by dilution (Table 3). Dietary protein, other nutrients, amino acids, vitamins, and minerals were maintained at constant levels so as not to be limiting.
Egg production (EP, %) was recorded daily, and egg weight (EW, g) was measured on three consecutive days each week. Egg mass (EM, g/bird·d) was determined using EP and EW. Feed leftovers were weighed weekly to quantify the weekly FI (g/bird·d). The feed conversion ratio (FCR, g/g) was calculated by dividing FI by EM and was corrected for mortality. Body weight (BW, g/bird) was measured at the first and tenth week of the assay. The crude fiber (CF) content of the feed or its indigestible portion was used as a measure of physical capacity intake or scaled feed intake (SFI, g/kg metabolic BW0.67).
At the end of the tenth week, excreta were collected using the total collection method to determine the MEn of the experimental diets. Ferric oxide (10 g/kg) was added to each treatment diet with proper mixing as a marker to define the beginning and end of the excreta collection period. The period of adaptation to diet was 72 h. The excreta were collected in adapted trays under the cages twice each day for three days, packed in plastic bags daily, and stored in a freezer (−20°C) until the end of the collection period when the samples were then homogenized per experimental unit, pre-dried, and ground in a ball mill. The diets and excreta samples were sent to the Laboratory of Poultry Sciences for analysis of dry matter (AOAC Official Method 930.15), total nitrogen (AOAC Official Method 2001.11), and crude energy (Model Parr 6200 oxygen bomb calorimeter), and MEn values were calculated. The CF of the diet was analyzed on a dosi-fiber machine using the AOAC Official Method 920.39.
At the end of the experiment, 54 birds were selected (one bird per replicate) to analyze the oviduct morphometry and liver histology. The birds were individually identified and euthanized after an eight-hour fasting period. Carbon dioxide was used in a transparent chamber to allow individual birds to be observed. The flow rate was maintained at approximately 20% of the chamber volume per min. The gas flow was maintained for at least 3 min after apparent clinical death. The chamber was sanitized after each euthanasia procedure.
The livers and oviducts were collected, measured using an electronic digital device, and weighed on a digital scale (0.001 g). The right and left lobes of the livers were separately weighed. The number of folds in each magnum and isthmus was quantified.
For analysis of the histological parameters of the liver, samples from the median region of the left lobe (approximately 1 cm2) were collected and fixed immediately in Bouin's solution for 24 h. They were then washed in alcohol (70%) to remove the fixative, dehydrated in a series of alcohols, diaphanized in xylol, and embedded in paraffin. Four semi-serial histological sections (7 μm thickness) were obtained from each bird and placed on the histological slides, and they were subsequently stained using the hematoxylin-eosin (HE) technique. Five fields were photographed randomly from each cut with the aid of a digital camera (Leica DFC 295) attached to a microscope (Leica-DM 2500), and the images were analyzed using LAS V.3.8 (Leica, Wetzlar, Germany).
Three photomicrographs per slide based on a randomization table were randomly chosen to determine variables. The areas of Kupffer cells and steatosis were measured [18]. To count Kupffer cells and steatosis, a grid with five frames (50×50 μm each) composed of dotted and continuous lines was superimposed onto each of the photos to randomize the counting area. Cell counting was performed on all frames (including cells on the dotted lines) while discarding those located on the continuous lines [18].
The data from the current experiment were analyzed using the PROC NLIN statement of SAS 9.4 (Statistical Analysis for Windows, SAS Institute Inc., Cary, NC, USA). The linear plateau models (one slope and two slopes) were adjusted according to previously described procedures [19].
The responses (ξ) were considered as dependent variables, and the mean levels were analyzed (X) as independent variables. A broken-line model was used with a slope (Eq. 1), quadratic (Eqs. 2), and two slopes or two lines (Eq. 3).
where (τ–X) is defined as zero when X>τ for the single-slope Eq. 1 and quadratic Eq. 2. The two-slope broken-line model expressed in Eq. 3, (τ–X) is defined as zero at X>τ, and (X–τ) is defined as zero when X<τ. We used the parameters for the breakpoint X value (τ), an asymptote for the first segment (ψ), and slopes for the 2 line segments (α, β). Parameters were estimated using the procedure described previously [19].
The morphometric and histological variables were subjected to factorial analysis that is a multivariate exploratory technique. Thirteen variables were used for this analysis, including BW, liver weight, liver length, right liver lobe weight, left liver lobe weight, oviduct weight, oviduct length, number of isthmus folds, number of ovary folds of magnum, number of Kupffer cells, number of steatoses, area of Kupffer cells, and area of steatosis. First, the adequacy of the sample space was analyzed through the correlation matrix, Kaiser-Meyer-Olkin test, Bartlett sphericity test, anti-image matrix, and commonalities [20]. The results led to the exclusion of the weight of the bird, number of folds of the isthmus, and number of Kupffer cells from the factorial analysis. Then, we used principal components as a method to extract the factors while considering that the value 1.00 is the minimum for an eigenvalue to be significant and for the load of a variable to be considered significant. The minimum value was 0.70 within one factor.
Factor analysis was used to create latent variables associated with the originally measured variables to thus allow analysis of variance considering several variables simultaneously to verify the statistical significance of the relationship between the latent variables classified within each factor and its relationship with the levels of MEn. Considering the means of extracting latent variables, we here may refer to multivariate analysis of variance.
In general, the various MEn levels modified the bird responses (p<0.010) for all variables of zootechnical performance (Table 4). Considering FI, none of the MEn levels were the result of birds consuming the full 27 g/d of feed offered. The level of MEn at 1,609 kcal/kg exhibited the highest FI of 25.7 g/d compared to the level of 3,250 kcal/kg for 23.3 g/d, and this represented a difference of 2.4 g. Comparing the results of FI and metabolizable energy intake (MEI) it was observed that at levels of 1,609, 1,740, and 1,895 kcal/kg, the birds consumed more feed and consumed less energy. For diets possessing a greater amount of fiber and as MEn levels increased, the FI decreased and the MEI increased.
According to Figure 1, the broken-line model with two slopes allowed for the description of the SFI that was closer to the mean contour of the treatment responses. The level possessing a higher CF content exhibited a reduction in the SFI. This reduction influenced the response of asymptote shaping when the broken-line model was adjusted with a slope according to the equation:
For X<τ and when X>τ, [τ–X] = 0. The reduction in FI as represented by the SFI verified at the level with the highest CF content was explained by the broken-line model with two slopes according to the following equation:
By definition, [186–X] = 0 when X>τ and [X–186] = 0 when X≤τ. The rate of reduction in the SF after 186 g/kg of CF in diet is 4.5 (0.1614/0.7276)-fold greater than is the rate that describes increasing SFI.
According to the model fitted for FI, the asymptote (ψ) in the FI occurred at 3,040 kcal/kg. According to our model, FI is expected to increase by 0.38% for every 100 kcal reduction in the diet (Table 5).
The lowest EP was 2.3% for the 1,609 kcal/kg level, and the highest EP was 77.8% for the 3,058 kcal/kg level. The asymptote (ψ) for EP was 74%, and this occurred from 2,829 kcal/kg according to the model presented in Table 5. According to this model, a reduction of 100 kcal/kg in the diet resulted in a 7.9% reduction in the EP.
There was a significant difference for the EW (Table 4), where the lowest EW was 10.2 grams (2,260 kcal/kg) and the highest EW was 11.7 g (3,058 kcal/kg). There was a linear increase in EW from 1,802 kcal/kg (Table 4). The two-slope model fitted to this variable is presented in Table 5.
As the birds increased their MEI, there was also an increase in EM. The smallest EM was 0.2 grams (1,609 kcal/kg), and the largest was 9.1 g (3,058 kcal/kg). The response asymptote (ψ) to EM stabilized at 8.44 g/bird·d, and this corresponded to the 2,965 kcal/kg level. According to the model (Table 5), a reduction of 100 kcal/kg in the diet causes the EM to become reduced by 7.0%.
Overall, there was an improvement in FCR as the levels of MEn in the diet increased (Table 4). The highest FCR (98.1± 38.6) was observed for the first level of MEn, and the lowest FCR was observed for the highest level of 3,058 kcal/kg for MEn. The model with a quadratic ascending fit to the variable FCR is presented in Table 5. According to this model, stabilization of the response (ψ) at 3.12 g/g occurred at a concentration of 2,611 kcal/kg.
The BW of the birds at the start of the trial varied from 184 to 188 g (a difference of 3.24 g), and these data are not provided in the tables. At the end of the assay, the weight of the birds varied from 153 to 194 (a 41 g difference) as presented in Table 4. The first four levels of MEn resulted in weight loss in the birds, and the largest losses were observed for the first two levels of MEn (average of 31.76 g). Weight loss decreased the extent to which the MEI was increased. Table 4 indicates a linear increase in the ΔBW of birds that occurred with an increase in the level of MEn in the diet until it reached the point where there was no further response (the response asymptote [ψ]). According to the model (Table 5), the point where the break (τ) occurred was 2,892 kcal/kg, and at this level the ΔBW close to zero (0.007 g).
The MEn levels did not significantly affect the morphometric variables of the liver and oviduct (Table 6) or the morphological variables of the liver (Table 7). In general, there was a numerical difference between the level of 1,609 kcal/kg and the low average values compared to the 3,059 kcal/kg level. The datasets presented in Tables 6 and 7 were subjected to an analysis of factors to evaluate the interrelationship between the variables (Table 8).
Table 8 presents the four factors obtained in the factorial analysis that was performed for the extracted parameters and the variables comprising each factor. There was no significant effect (p>0.050) for energy level on the parameters that were evaluated by variance analysis (Table 8). The analysis of variables that correlate with each of the factors revealed that factor 1, factor 2, and factor 3 exhibited a positive correlation among a group of variables. Factor 1 indicated that when the weight of the liver was increased, the right lobe weight, left lobe weight, and the number of steatoses increased. Factor 2 indicated that when the weight was increased, the oviduct became increased in length. Factor 3 indicated that when the number of pleats in the magnum was increased, the length of the liver became increased, and factor 4 revealed a negative correlation between Kupffer cells and steatosis cells. This indicated that when the area of the Kupffer cells was increased, the area of the steatosis cells was decreased.
To obtain range of responses using the dose-response method, the evaluated nutrient must be limited. To achieve this, we applied the concept of “theoretical” dilution technique [15]. According to the results obtained for EP and EM, MEn was the most limiting nutritional resource for the validation of the study. This can be verified by the theory of FI regulation that was proposed previously [21] and that states that if the diet is not properly balanced, the bird will increase its FI in an attempt to compensate for the most limiting nutritional resource. We have observed this in our current study as evidenced by the ratio of 0.38% for each reduction of 100 kcal in the diet. This rate is expected to continue to increase. Although it has not been measured, it is speculated that the physicochemical characteristics of the ingredients used in the diluted diet may have attenuated the increase in FI by providing greater filling of the crop.
This physical capacity intake must be measured in terms of feed characteristics such as CF, density, and water-holding capacity, and the appropriate parameter must be measured in feed ingredients [22]. Although the birds used this artifice under adverse nutrient conditions, reaching the level of 121 g/kg of BW0.67, was only possible under conditions of term neutrality. This is a condition offered in systems with controlled environments. The recurrence of the use of this ingestion capacity in controlled environments may be related to birds with desirable BW. The quest to regulate FI (the limiting nutrient) inevitably generates excess intake from other routes, and the means by which to store the excess carbon from the constitution of amino acids, fatty acids, and carbohydrates is in the form of body fat. Therefore, it is reasonable to assume that Japanese quail production can regulate FI according to limiting nutrient theory.
An improvement in EP of 2.3% to 77.8% and of EM from 0.2 to 9.1 (g/bird·d) was observed with an increase in the MEn level. This result demonstrates the importance of understanding the nutritional requirements of MEn to achieve the maximum performance potential of these birds. The level of 1,609 kcal/kg presented an EP that was 2.3% lower than that of the treatments, and this explains why the birds were close to the physiological state of maintenance. Moreover, it was observed that 0.57 g of tissue per day was mobilized from body reserve for that purpose. Therefore, the instinct for reproduction was preserved. A similar description was not found in the literature.
Regarding EW, the increasing levels of MEn led to an increase in weight (1.5 g), possibly due to the increase in MEn daily intake being sufficient to meet the production requirements and to obtain heavier eggs. The primary factor that affects EW is BW [1–3]. This was observed in this study, as the birds with slight eggs exhibited a BW loss of 41 g (in the period) compared to that at the levels of 1,609 and 3,050 kcal/kg.
The birds that received diets below 2,394 kcal/kg of MEn presented with FCR due to the MEn dilution of diets and the low EP at those levels. A previous study [5] verified the improvement in FCR with increased MEn. Although in most surveys evaluating the effects of various levels of MEn on quail laying, there have been no observed significant effects in regard to FCR. One factor that can likely explain the absence of effects is the levels that are evaluated, as the lowest level in this study was 1,609 kcal/kg, while in the published works we found that the lowest level was 2,500 kcal/kg [12,13].
The linear increase in the BW of birds can be attributed to the increase in MEI, and this variable has been previously correlated with the variation in BW [1,2]. The results of this research reveal the importance of understanding the energy requirements of Japanese quails during the laying phase.
The levels of MEn used did not influence the morphological parameters of the liver and oviduct or the histology of the liver, thus indicating that levels between 1,609 and 3,050 kcal/kg do not affect the morphometry of the liver or the oviduct. It was expected that birds fed with levels above the recommended levels [23,17] at 2,800 and 2,900 kcal/kg, respectively, would develop steatosis or nutritional diseases such as fatty liver syndrome. A survey reported nutritional disease in Japanese quails but did not report any involvement with MEn levels [24]. However, it has been reported that the relationship between protein and physical activity is a factor that predisposes commercial layers to the appearance of fatty liver [25,26], and quail laying was not determined to support placement based on the literature. Based on this scenario, multivariate analysis techniques were used to maximize relevant data collection. The analysis generated four factors (commonly called processes), and in this study, we refer to these factors as the physiological process. By exploring factor 1 or physiological process contained in factor 1, there is an indication that the increase in the weight of the liver leads to an increase in the weight of the right and left lobe and the area of steatosis, although this increase was not significant. From a physiological point of view, it is expected that this process would be associated with the levels of Men when considering that the liver performs several functions between the storage of carbohydrates and fats [16]. This activity can induce an increase in the weight of the liver (referring to steatosis) and can also be applied based on the knowledge that lipidosis or hepatic steatosis may be derived from several factors ranging from nutritional factors to poisoning cases.
Once the physiological process has been detected, the limitation of this research lies in the attribution of its cause. It is speculated that the lack of significance for a number of our data points was due to the reduced number of birds sampled for this analysis. Therefore, future surveys should use larger sample sizes.
The physiological process contained in factor 2 grouped the variables related to the oviduct, weight, and length, and it exhibited an allometric relation. When the weight was increased, the length became increased. Although they are orthogonal, factor 3 and factor 4 grouped histological variables of the liver demonstrated that when Kupffer cells increase steatosis, this can be explained by the increase in Men. Additionally, the presence of vacuoles in the hepatocytes increases. This association reveals the natural mechanisms used to store energy. Kupffer cells form the major portion of the endothelial reticulum or mononuclear phagocyte system [27], where an excessive increase in steatosis maintains a physiological balance of energy and thus avoids metabolic diseases.
Notes
ACKNOWLEDGMENTS
The authors would like to thank the PEC-PG program for granting the doctoral scholarship and Paulo Renê Silva-Júnior for the valuable discussions on this manuscript.
Figure 1
The mean relationship between the scaled feed intake (SFI) of Japanese quails at the laying stage (g feed/kg metabolic body weight/d) and crude fiber content in the feed (g/kg).
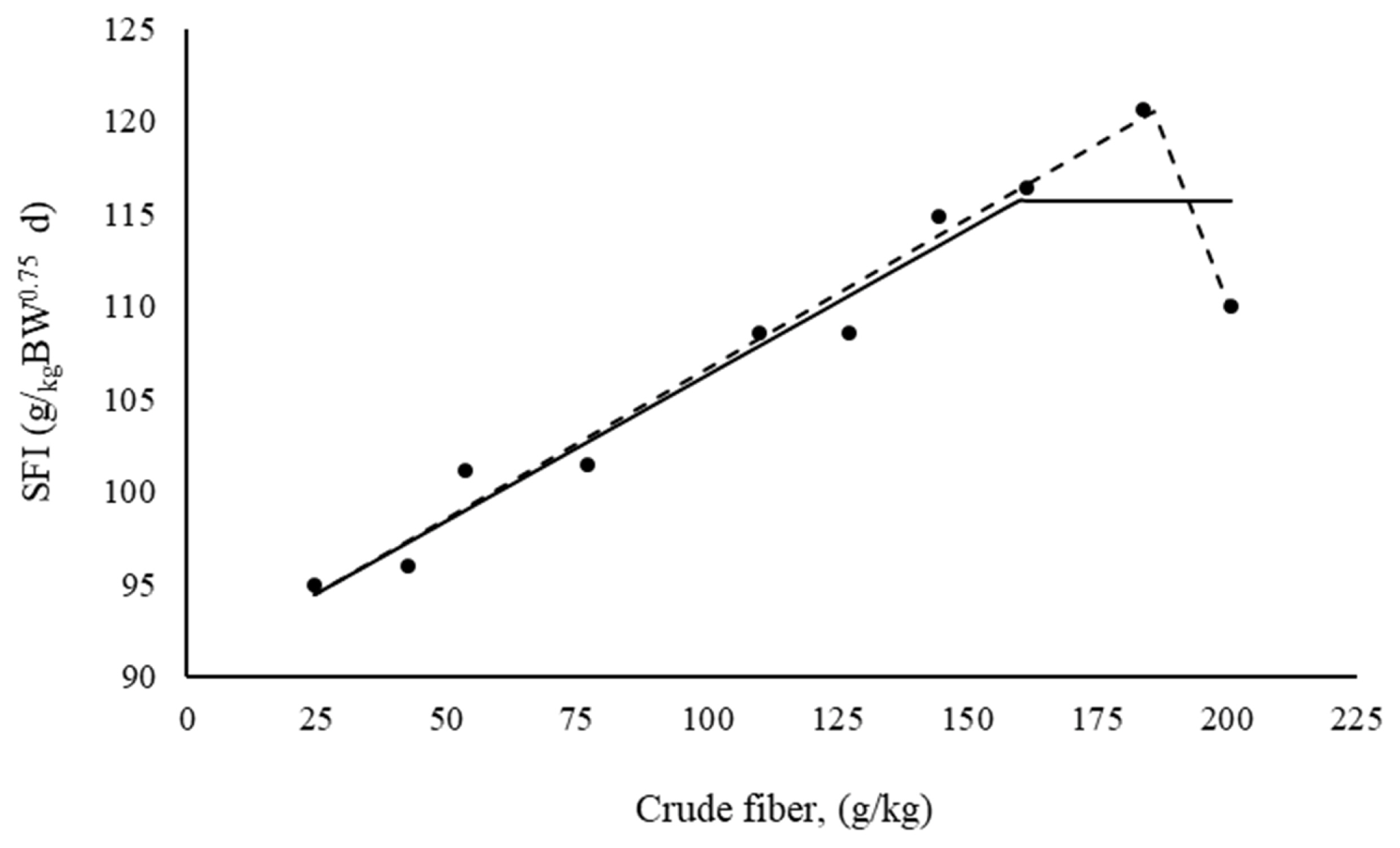
Table 1
Percentual (%) and nutritional composition as calculated (%) from experimental diets
| Items | Low 1,500 | Level of metabolizable energy (kcal/kg) | High 3,250 | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| 1,890 | 2,060 | 2,230 | 2,400 | 2,730 | 2,960 | 3,070 | |||
| Ingredients | |||||||||
| Corn | 16.16 | 24.95 | 28.78 | 32.61 | 36.44 | 43.87 | 49.06 | 51.53 | 55.59 |
| Soybean meal 45% | 32.91 | 31.05 | 30.23 | 29.42 | 28.61 | 27.03 | 25.94 | 25.41 | 24.55 |
| Rice husk | 23.11 | 17.96 | 15.71 | 13.47 | 11.22 | 6.87 | 3.83 | 2.38 | - |
| Soy oil | 0.7 | 2.18 | 2.83 | 3.48 | 4.13 | 5.38 | 6.26 | 6.67 | 7.36 |
| Lignocellulose 65% | 15.00 | 11.66 | 10.20 | 8.74 | 7.29 | 4.46 | 2.49 | 1.54 | - |
| Limestone | 6.72 | 6.72 | 6.73 | 6.73 | 6.73 | 6.73 | 6.74 | 6.74 | 6.74 |
| Dicalcium phosphate | 1.16 | 1.16 | 1.16 | 1.16 | 1.16 | 1.16 | 1.16 | 1.16 | 1.16 |
| Salt | 0.28 | 0.29 | 0.30 | 0.31 | 0.31 | 0.32 | 0.33 | 0.33 | 0.34 |
| DL-Methionine, 99% | 0.86 | 0.85 | 0.84 | 0.84 | 0.83 | 0.82 | 0.82 | 0.82 | 0.81 |
| L-Lysine HCl, 78% | 0.6 | 0.63 | 0.65 | 0.66 | 0.68 | 0.71 | 0.73 | 0.73 | 0.75 |
| L-Arginine | 0.72 | 0.74 | 0.76 | 0.77 | 0.78 | 0.80 | 0.81 | 0.82 | 0.83 |
| L-Threonine | 0.39 | 0.39 | 0.40 | 0.40 | 0.40 | 0.40 | 0.41 | 0.41 | 0.41 |
| L-Valine | 0.48 | 0.48 | 0.48 | 0.48 | 0.49 | 0.49 | 0.49 | 0.49 | 0.49 |
| L-Isoleucine | 0.36 | 0.37 | 0.37 | 0.38 | 0.38 | 0.39 | 0.39 | 0.40 | 0.40 |
| L-Tryptophan | 0.14 | 0.14 | 0.15 | 0.15 | 0.15 | 0.15 | 0.16 | 0.16 | 0.16 |
| Vitamin and Mineral premix1) | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| BHT2) | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Nutritional composition calculated | |||||||||
| Metabolizable energy (kcal/kg) | 1,500 | 1,890 | 2,060 | 2,230 | 2,400 | 2,730 | 2,960 | 3,070 | 3,250 |
| Crude protein (%) | 19.50 | 19.50 | 19.50 | 19.50 | 19.50 | 19.50 | 19.50 | 19.50 | 19.50 |
| Digestible lysine (%) | 1.30 | 1.30 | 1.30 | 1.30 | 1.30 | 1.30 | 1.30 | 1.30 | 1.30 |
| Digestible M+C (%) | 1.24 | 1.24 | 1.24 | 1.24 | 1.24 | 1.24 | 1.24 | 1.24 | 1.24 |
| Digestible threonine (%) | 0.91 | 0.91 | 0.91 | 0.91 | 0.91 | 0.91 | 0.91 | 0.91 | 0.91 |
| Digestible tryptophan (%) | 0.31 | 0.31 | 0.31 | 0.31 | 0.31 | 0.31 | 0.31 | 0.31 | 0.31 |
| Digestible valine (%) | 1.13 | 1.13 | 1.13 | 1.13 | 1.13 | 1.13 | 1.13 | 1.13 | 1.13 |
| Calcium (%) | 2.90 | 2.90 | 2.90 | 2.90 | 2.90 | 2.90 | 2.90 | 2.90 | 2.90 |
| Available phosphorus (%) | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Sodium (%) | 0.14 | 0.14 | 0.14 | 0.14 | 0.14 | 0.14 | 0.14 | 0.14 | 0.14 |
1) Vitamin premix provided the following (per kg of diet): Vit. A 1,750.000 U. I; Vit. D3 500,000 U. I; Vit. E 2,000 U. I; Vit. K3 500 mg; Vit. B1 250 mg; Vit. B2 875 mg; Vit. B6 500 mg; Vit. B12 1,250 mcg/kg; niacin 6,250 mg; choline 65 g; pentatonic acid 2,500 mg; copper 2,000 mg/kg; ferro 12,500 g; manganese 17,500 g; zinc 12,500 g; iodine 300 mg; selenium 50 mg.
Table 2
Nutritional requirement of Japanese quail during the laying phase according to Silva et al [17]
Table 3
Dilution levels and metabolizable energy used in the Japanese quail during the laying phase assay
Table 4
Means (±standard error) of the responses and mortality of Japanese quails during the laying phase when subjected to different dietary concentrations of metabolizable energy
| Levels kcal/kg | FI (g/bird·d) | EP (%/bird/d) | EW (g) | EO (g/d) | FCR (g/g) | BW (g) | ΔBW (g/bird·d gain) | MOR | |
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Calculated | Analyzed | ||||||||
| 1,500 | 1,609 | 25.7±2.2 | 2.3±1.3 | 10.8±0.8 | 0.2±0.1 | 98.1±38.6 | 53±6 | −0.57±0.15 | 8 |
| 1,890 | 1,740 | 23.9±1.4 | 10.1±1.4 | 10.3±0.6 | 1.0±0.2 | 23.2±3.5 | 155±8 | −0.35±0.08 | 1 |
| 2,060 | 1,895 | 23.5±1.3 | 19.1±3.9 | 10.8±0.4 | 2.1±0.4 | 12.0±2.8 | 166±5 | −0.25±0.04 | 4 |
| 2,230 | 2,260 | 24.4±0.7 | 29.4±12.6 | 10.2±0.4 | 3.0±1.5 | 8.7±2.4 | 169±6 | −0.16±0.05 | 1 |
| 2,400 | 2,394 | 24.2±0.5 | 50.0±16.2 | 10.8±0.4 | 5.4±1.6 | 4.5±1.0 | 175±13 | −0.15±0.07 | 2 |
| 2,730 | 2,643 | 23.8±1.9 | 69.4±6.3 | 11.2±0.2 | 7.8±0.8 | 3.2±0.2 | 180±14 | −0.04±0.06 | 1 |
| 2,960 | 2,892 | 23.5±0.7 | 73.9±2.2 | 11.2±0.3 | 8.2±0.4 | 2.9 ±0.1 | 181±9 | 0.01±0.07 | 1 |
| 3,070 | 3,045 | 23.4±0.7 | 75.1±6.2 | 11.3±0.3 | 8.5±0.8 | 2.8±0.2 | 190±8 | 0.08±0.02 | 0 |
| 3,250 | 3,058 | 23.3±0.6 | 77.8±3.3 | 11.7±0.6 | 9.1±0.3 | 2.6±0.1 | 194±12 | 0.17±0.02 | 0 |
| Average | 24.0 | 45.2 | 10.9 | 5.0 | 17.6 | 163 | −0.14 | 2 | |
| SEM | 0.103 | 4.176 | 0.066 | 0.483 | 4.251 | - | 0.031 | - | |
| p-value1) | 0.026 | 0.001 | 0.001 | 0.001 | 0.001 | - | 0.001 | - | |
Table 5
Parameters fitted for the responses of Japanese quails during the laying phase when subjected to different concentrations of metabolizable energy in the diet
| Parameters | FI (g/bird·d) | EP (%/bird/d) | EW | EO (g/d) | FCR (g/g) | ΔBW (g/bird·d gain) |
|---|---|---|---|---|---|---|
| ψ, Asymptote, Y axis | 23.4±0.43 | 74.0±2.09 | 10.4±0.14 | 8.4±0.34 | 3.1±0.49 | 0.007±0.0007 |
| α, Slope | −0.9±0.53 | 58.2±4.40 | 1.9 ±0.21 | 5.9± 0.41 | −25.2±5.23 | −0.317±0.075 |
| τ, Break point, X axis | 3,040±72 | 2,820±7 | 1,802±180 | 2,960± 8 | 2,610±87 | 2,892±282 |
| β, Slope | - | - | 0.78±0.12 | - | - | - |
| p-value1) | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 |
| R2 | 51.85 | 90.59 | 30.21 | 86.49 | 89.86 | 25.32 |
Table 6
Average (±standard error) of morphological and histological parameters of the liver and oviduct of laying quails subjected to different levels of energy
| Levels (kcal/kg) | BW (g) | LW (g) | LL | RLW (g) | LLW (g) | OW (g) | OL (cm) | NMC | NIC | |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Calculated | Analyzed | |||||||||
| 1,500 | 1,609 | 145±19 | 2.4±0.1 | 3.7±0.1 | 1.2±0.2 | 0.7±0.1 | 0.5±0.2 | 6.4±4.4 | 11.0±0.1 | 18.0±0.1 |
| 1,890 | 1,740 | 161±80 | 5.0±0.9 | 4.9±0.7 | 3.0±0.5 | 1.9±0.5 | 5.5±1.3 | 24.6±7.7 | 14.2±1.2 | 13.6±1.8 |
| 2,060 | 1,895 | 164±15 | 4.7±1.2 | 4.1±0.4 | 2.8±0.5 | 1.8±0.5 | 5.3±0.8 | 29.5±2.6 | 14.8±2.2 | 15.6±0.8 |
| 2,230 | 2,260 | 169±17 | 5.2±1.3 | 4.1±0.3 | 3.0±0.8 | 1.9±0.3 | 5.6±1.1 | 28.7±2.4 | 13.0±1.1 | 13.8±1.2 |
| 2,400 | 2,394 | 170±16 | 5.3±1.0 | 4.6±0.4 | 2.8±1.0 | 2.4±0.4 | 6.4±0.7 | 33.0±2.7 | 14.2±1.5 | 14.7±1.5 |
| 2,730 | 2,643 | 174±10 | 4.2±1.4 | 3.5±0.7 | 2.5±0.9 | 1.8±0.8 | 5.2±1.6 | 28.9±3.3 | 13.2±1.1 | 14.0±1.4 |
| 2,960 | 2,892 | 178±80 | 4.8±0.7 | 4.3±0.2 | 2.8±0.4 | 1.9±0.3 | 6.3±0.9 | 34.0±2.8 | 14.8±0.7 | 13.4±1.5 |
| 3,070 | 3,045 | 180±21 | 5.6±0.5 | 4.1±0.2 | 3.4±0.4 | 2.2±0.4 | 7.1±1.2 | 29.2±5.6 | 12.8±2.7 | 12.6±1.8 |
| 3,250 | 3,058 | 182±13 | 6.6±0.4 | 4.3±0.3 | 3.8±0.3 | 2.6±0.4 | 7.5±1.3 | 31.5±4.0 | 15.0±0.6 | 14.4±1.8 |
| Average | 169 | 4.9 | 4.2 | 2.8 | 1.9 | 5.5 | 27.3 | 13.7 | 14.5 | |
| SEM | 1.57 | 0.16 | 0.06 | 0.10 | 0.07 | 0.28 | 1.13 | 0.18 | 0.21 | |
| p-value | NS1) | NS | NS | NS | NS | NS | NS | NS | NS | |
Table 7
Means (±standard errors) for number of steatosis cells, number of Kupffer cells, steatosis area, and Kupffer cell area of Japanese quail during the laying phase
Table 8
Analysis of morphological and histological parameters of the liver and oviduct of laying quails subjected to different levels of energy
| Variables | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| Liver weight | 0.961* | 0.089 | 0.050 | 0.013 |
| Liver length | 0.062 | −0.118 | 0.732* | −0.135 |
| Right lobe weight | 0.765* | −0.137 | 0.006 | −0.002 |
| Left lobe weight | 0.754* | 0.286 | 0.009 | 0.046 |
| Oviduct weight | 0.130 | 0.756* | 0.320 | 0.002 |
| Oviduct length | −0.006 | 0.830* | −0.163 | −0.017 |
| Number of magno’s crimp | 0.049 | 0.143 | 0.795* | 0.163 |
| Esteatosis number | 0.697* | −0.237 | 0.189 | −0.280 |
| Esteatosis area | 0.344 | −0.269 | 0.274 | −0.761* |
| Kupffer cells area | 0.206 | −0.234 | 0.281 | 0.816* |
| Explained variance | 2.751 | 1.589 | 1.492 | 1.372 |
| p-value1) | 0.148 | 0.500 | 0.148 | 0.937 |
REFERENCES
1. Silva EP, Sakomura NK, Sarcinelli MF, Dorigam JCP, Venturini KS, Lima MB. Modeling the response of Japanese quail hens to lysine intake. Livest Sci 2019; 224:69–74.
https://doi.org/10.1016/j.livsci.2019.04.005

2. Sarcinelli MF, Sakomura NK, Dorigam JCP, et al. Modelling Japanese quail responses to methionine + cystine, threonine and tryptophan intake. Anim Feed Sci Technol 2020; 263:114486
https://doi.org/10.1016/j.anifeedsci.2020.114486

3. Silva EP, Castiblanco DMC, Artoni SMB, Lima MB, Nogueira HS, Sakomura NK. Metabolisable energy partition for Japanese quails. Animal 2020; 14:s275–85.
https://doi.org/10.1017/S1751731120001445


4. Belo MTS, Cotta JTB, Oliveira AIG. Influence of dietary energy levels on laying japanese quails (Coturnix coturnix japonica). Sci Agrot 2000; 24:782–93.
5. Pinto R, Ferreira AS, Albino LFT, Gomes PC, Vargas JG. Protein and energy levels for laying japanese quails. R Bras Zootec 2002; 31:1761–70.
https://doi.org/10.1590/S1516-35982002000700019

6. Freitas AC, Fuentes MFF, Freitas ER, Sucupira FS, Oliveira BCM. Dietary crude protein and metabolizable energy levels on laying quails performance. R Bras Zootec 2005; 34:838–46.
https://doi.org/10.1590/S1516-35982005000300015

7. Lopes IRV, Fuentes MFF, Freitas ER, Soares MB, Ribeiro OS. Effect of cage density and metabolizable energy level of the diet on performance of Japanese quails. Cienc Agron 2006; 37:369–75.
8. Barreto SLT, Quirino BJS, Brito CO, et al. Metabolizable energy levels for Japanese quails in the initial laying phase. R Bras Zootec 2007; 36:79–85.
https://doi.org/10.1590/S1516-35982007000100010

9. Barreto SLT, Quirino BJS, Brito CO, et al. Effects of energy nutritional levels on performance and egg quality of European quails in the initial laying phase. R Bras Zootec 2007; 36:86–93.
https://doi.org/10.1590/S1516-35982007000100011

10. Moura GS, Barreto SLT, Donzele JL, Hosoda LR, Pena GM, Angelini MS. Diets of different energetic densities, keeping constant the metabolizable energy: nutrients ratio, for laying Japanese quails. R Bras Zootec 2008; 37:1628–33.
https://doi.org/10.1590/S1516-35982008000900015

11. Cavalcante LE, Costa FGP, Lima RC, et al. Determination of the metabolizable energy and crude protein relationship on the performance of japanese quails in the production phase. Rev Cien Prod Anim 2010; 12:166–8.
https://doi.org/10.15528/2176-4158/rcpa.v12n2p166-168

12. Hurtado-Nery VL, Torres NDM, Castro RAS. Effect of levels of metabolizable energy and protein on the zootechnical performance of laying quails. Cienc Agric 2014; 11:9–16.
https://doi.org/10.19053/01228420.3832

13. Hurtado-Nery VL, Torres NDM, Daza GMF. The effect of crude protein and metabolisable energy levels on quail egg quality. Orinoquia 2015; 19:195–202.

14. Agboola AF, Omidiwura BRO, Ologbosere EY, Iyayi EA. Determination of crude protein and metabolisable energy of japanese quail (Coturnix coturnix japonica) during laying period. J World Poult Res 2016; 6:131–8.
15. Gous RM, Griessel M, Morris TR. Effect of dietary energy concentration on the response of laying hens to amino acids. Br Poult Sci 1987; 28:427–36.
https://doi.org/10.1080/00071668708416977


16. Artoni SMB, Carneiro APM, Giacomini G, Moraes VMB, Araújo CSS, Araújo LF. Macroscopic and morphometric evaluation of japanese quail (Coturnix coturnix japonica) oviduct when fed diets with different protein levels. Braz J Poult Sci 2001; 3:225–31.
https://doi.org/10.1590/S1516-635X2001000300004

17. Silva JHV, Jordão Filho J, Costa FGP, Lacerda PB, Vargas DGV, Lima MR. Nutritional requirements of quails. Rev Bras Saúde Prod Anim 2012; 13:775–90.
https://doi.org/10.1590/S1519-99402012000300016

18. Gundersen HJG. Notes on the estimation of the numerical density of arbitrary profiles: the edge effect. J Microsc 1977; 111:219–23.
https://doi.org/10.1111/j.1365-2818.1977.tb00062.x

19. Robbins KR, Saxton AM, Southern LL. Estimation of nutrient requirements using broken-line regression analysis. J Anim Sci 2006; 84:155–65.
https://doi.org/10.2527/2006.8413_supple155x

20. Kaiser HF. The varimax criterion for analytic rotation in factor analysis. Psychometrika 1958; 23:187–200.
https://doi.org/10.1007/BF02289233

21. Emmans GC. A method to predict the food intake of domestic animals from birth to maturity as a function of time. J Theor Biol 1997; 186:189–99.
https://doi.org/10.1006/jtbi.1996.0357

22. Moore ML. The constraining effect of feed bulk on the voluntary feed intake of laying hens [master's thesis]. Pietermaritzburg KN, editorSouth Africa: South Africa University of KwaZulu-Natal; 2002.
23. Brazilian Tables for Poultry and Swine. 3rd edViçosa, Brazil: Universidade Federal de Viçosa; 2017.
24. Spurlock ME, Savage JE. Effect of dietary protein and selected antioxidants on fatty liver hemorrhagic syndrome induced in Japanese quail. Poult Sci 1993; 72:2095–105.
https://doi.org/10.3382/ps.0722095


25. Jiang S, Hester PY, Hu JY, Yan FF, Dennis RL, Cheng HW. Effect of perches on liver health of hens. Poult Sci 2014; 93:1618–22.
https://doi.org/10.3382/ps.2013-03659


26. Jiang S, Cheng HW, Cui LY, Zhou ZL, Hou JF. Changes of blood parameters associated with bone remodeling following experimentally induced fatty liver disorder in laying hens. Poult Sci 2013; 92:1443–53.
https://doi.org/10.3382/ps.2012-02800


27. Gandhi CH. Molecular pathology of liver diseases. Monga SPS, editorKupffer cells. Boston, MA, USA: Springer; 2011. p. 53–79.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





