 |
 |
| Anim Biosci > Volume 36(6); 2023 > Article |
|
Abstract
Objective
Methods
Results
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
FUNDING
This research was funded with the support of ŌĆ£Cooperative Research Program for Agriculture Science & Technology Development (Project title: Analysis of inherited traits and establishment of reproductive techniques for Korean native goats, Project No. PJ 01431501)ŌĆØ and 2021 the Academy-Research-Industry Support Program of the National Institute of Animal Science, Rural Development Administration, Republic of Korea.
Figure┬Ā1
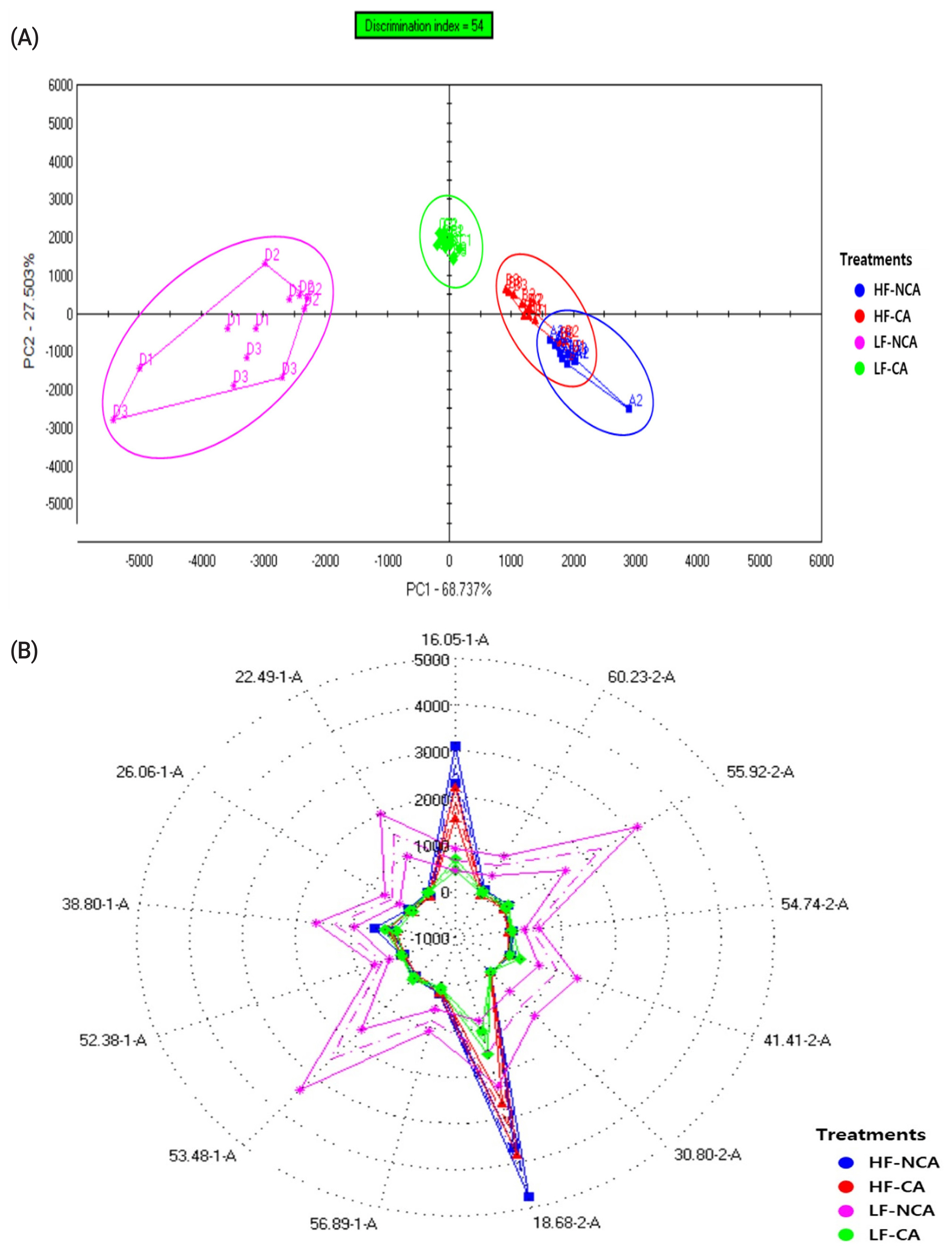
Figure┬Ā2
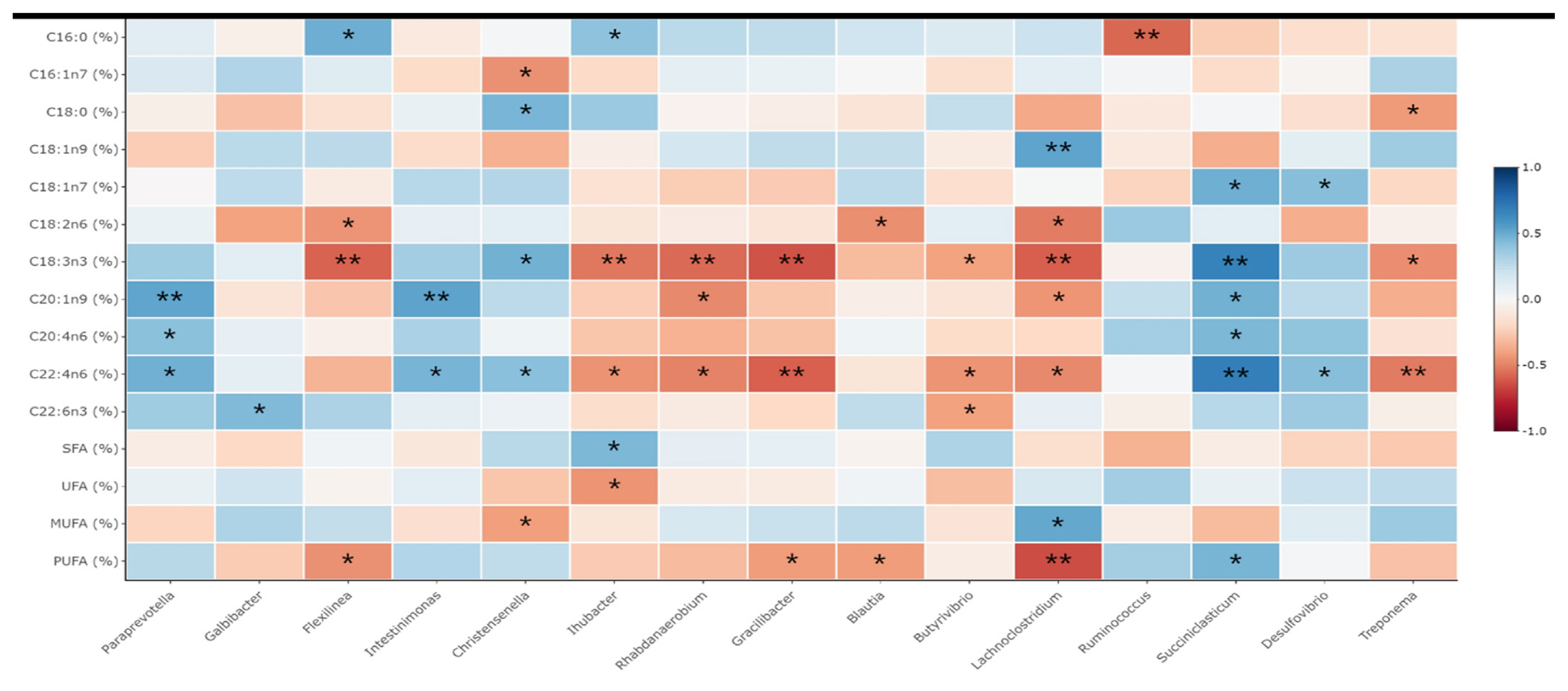
Table┬Ā1
| Variables | High forage | Low forage | SEM | p-value | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| CA | NCA | CA | NCA | D | G | D├ŚG | ||
| Proximate composition | ||||||||
| ŌĆāMoisture (%) | 73.31b | 76.25a | 74.43b | 76.36a | 0.41 | 0.152 | <0.001 | 0.237 |
| ŌĆāCrude protein (%) | 20.66 | 21.36 | 20.93 | 21.40 | 0.28 | 0.592 | 0.053 | 0.701 |
| ŌĆāCrude fat (%) | 5.44a | 3.51b | 5.72a | 3.46b | 0.18 | 0.525 | <0.001 | 0.366 |
| ŌĆāCrude ash (%) | 1.08a | 1.07a | 1.01b | 0.96b | 0.02 | 0.001 | 0.297 | 0.464 |
| Meat color | ||||||||
| ŌĆāCIE L | 40.42 | 40.41 | 41.00 | 39.81 | 0.73 | 0.991 | 0.423 | 0.430 |
| ŌĆāCIE a | 24.30 | 24.51 | 25.46 | 23.95 | 0.47 | 0.529 | 0.185 | 0.081 |
| ŌĆāCIE b | 13.69ab | 13.38b | 14.38a | 13.29b | 0.30 | 0.327 | 0.030 | 0.213 |
| Physicochemical characteristics | ||||||||
| ŌĆāpH | 6.03 | 5.93 | 5.94 | 5.95 | 0.04 | 0.278 | 0.204 | 0.140 |
| ŌĆāWater holding capacity (%) | 51.04a | 46.55b | 47.55b | 45.86b | 1.18 | 0.092 | 0.017 | 0.249 |
| ŌĆāCooking loss (%) | 32.66 | 34.66 | 32.27 | 34.65 | 1.31 | 0.880 | 0.109 | 0.888 |
| ŌĆāShear force (kgf) | 6.79 | 7.21 | 6.71 | 6.92 | 0.16 | 0.249 | 0.059 | 0.520 |
| ŌĆāAPC (log CFU/g) | 2.30 | 2.37 | 2.23 | 2.28 | 0.06 | 0.171 | 0.358 | 0.856 |
| ŌĆāVBN (mg/100 g) | 6.76 | 7.03 | 7.00 | 6.87 | 0.23 | 0.869 | 0.767 | 0.415 |
Table┬Ā2
| Variables (%) | High forage | Low forage | SEM | p-value | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| CA | NCA | CA | NCA | D | G | D├ŚG | ||
| C14:0 (myristic acid) | 1.78b | 2.29a | 1.75b | 2.27a | 0.13 | 0.831 | 0.001 | 0.988 |
| C16:0 (palmitic acid) | 23.02 | 24.23 | 24.00 | 24.73 | 0.63 | 0.254 | 0.141 | 0.712 |
| C16:1n7 (palmitoleic acid) | 1.44ab | 1.18b | 1.71a | 1.28b | 0.11 | 0.124 | 0.007 | 0.460 |
| C18:0 (stearic acid) | 15.72b | 18.27a | 14.44b | 17.43a | 0.55 | 0.066 | <0.001 | 0.693 |
| C18:1n9 (oleic acid) | 32.68b | 28.63c | 38.56a | 30.96bc | 0.93 | <0.001 | <0.001 | 0.072 |
| C18:1n7 (vaccenic acid) | 2.27a | 1.85ab | 1.60b | 1.45b | 0.18 | 0.008 | 0.130 | 0.481 |
| C18:2n6 (linoleic acid) | 10.29bc | 12.50ab | 8.81c | 13.26a | 0.79 | 0.654 | <0.001 | 0.170 |
| C18:3n6 (╬│-linoleic acid) | 0.11ab | 0.08b | 0.16a | 0.08b | 0.02 | 0.233 | 0.015 | 0.265 |
| C18:3n3 (╬▒-linolenic acid) | 1.83a | 2.11a | 0.59b | 0.69b | 0.14 | <0.001 | 0.196 | 0.536 |
| C20:1n9 (eicosenoic acid) | 0.36ab | 0.47a | 0.28b | 0.34ab | 0.05 | 0.036 | 0.105 | 0.580 |
| C20:4n6 (arachidonic acid) | 7.21a | 5.94b | 6.08b | 5.56b | 0.37 | 0.056 | 0.026 | 0.316 |
| C20:5n3 (eicosapentaenoic acid) | 0.41a | 0.25b | 0.34ab | 0.40ab | 0.05 | 0.420 | 0.309 | 0.037 |
| C22:4n6 (adrenic acid) | 2.56a | 2.08a | 1.50b | 1.44b | 0.16 | <0.001 | 0.125 | 0.216 |
| C22:6n3 (docosahexaenoic acid) | 0.32a | 0.12b | 0.18b | 0.12b | 0.03 | 0.033 | <0.001 | 0.031 |
| SFA | 40.52b | 44.79a | 40.18b | 44.43a | 1.00 | 0.731 | <0.001 | 0.988 |
| UFA | 59.48a | 55.21b | 59.80a | 55.57b | 1.00 | 0.738 | <0.001 | 0.980 |
| MUFA | 36.76b | 32.14c | 42.16a | 34.02c | 0.91 | 0.001 | <0.001 | 0.068 |
| PUFA | 22.73a | 23.07a | 17.64b | 21.56a | 1.29 | 0.019 | 0.115 | 0.183 |
| MUFA/SFA | 0.91b | 0.72c | 1.05a | 0.77c | 0.03 | 0.006 | <0.001 | 0.107 |
| PUFA/SFA | 0.57 | 0.52 | 0.44 | 0.49 | 0.04 | 0.071 | 0.997 | 0.294 |
| n6/n3 ratio | 8.11b | 8.42b | 14.99a | 16.94a | 0.67 | <0.001 | 0.109 | 0.239 |
CA, castration; NCA, non-castration; SEM, standard error of the means; D, diet; G, gender; I, interaction; SFA, saturated fatty acids; UFA, unsaturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; n6, fatty acid with the last double bond at 6th carbon from the methyl end; n3, fatty acid with the last double bond at 3rd carbon from the methyl end.
Table┬Ā3
| Compounds | Sensory description | Column | RT (RI) | High forage diets | Low forage diets | SEM | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||
| CA | NCA | CA | NCA | D | G | D├ŚG | |||||
| Dichloromethane | ND | MXT-5 | 16.05 (505) | 1,905.4b | 2,714.2a | 564.3c | 665.7c | 88.2 | <0.001 | <0.001 | <0.001 |
| MXT-1701 | 18.68 (611) | 3,234.6b | 4,206.9a | 1,331.7c | 1,576.1c | 165.6 | <0.001 | 0.001 | 0.033 | ||
| Methyl propanoate | Etheral, fruity, rum | MXT-5 | 22.49 (627) | 26.3b | 56.8b | 91.2b | 1,493.0a | 78.6 | <0.001 | <0.001 | <0.001 |
| MXT-1701 | ND | ND | ND | ND | ND | - | - | - | - | ||
| 1-Hydroxy-2-propanone | Caramelized, sweet | MXT-5 | 26.06 (664) | 15.3b | 43.3b | 14.1b | 437.7a | 25.6 | <0.001 | <0.001 | <0.001 |
| MXT-1701 | ND | ND | ND | ND | ND | - | - | - | - | ||
| 1-Propanol, 2-methyl- | Alcoholic, bitter, glue, leek | MXT-5 | ND | ND | ND | ND | ND | - | - | - | - |
| MXT-1701 | 30.80 (745) | 0.0b | 0.0b | 0.0b | 906.5a | 53.6 | <0.001 | <0.001 | <0.001 | ||
| [E]-2-penten-1-ol | Grassy, green, mushroom | MXT-5 | 38.80 (769) | 196.9b | 375.8b | 238.5b | 1,274.1a | 63.3 | <0.001 | <0.001 | <0.001 |
| MXT-1701 | ND | ND | ND | ND | ND | - | - | - | - | ||
| 2,4-Octadiene | Glue, warm | MXT-5 | ND | ND | ND | ND | ND | - | - | - | - |
| MXT-1701 | 41.41 (825) | 93.3b | 108.3b | 196.0b | 1,070.8a | 61.3 | <0.001 | <0.001 | <0.001 | ||
| Chlorobenzene | Etheral, floral, sweet | MXT-5 | 52.38 (866) | 59.1b | 53.4b | 83.8b | 481.3a | 23.9 | <0.001 | <0.001 | <0.001 |
| MXT-1701 | 54.74 (920) | 18.7b | 55.2b | 70.3b | 445.1a | 22.7 | <0.001 | <0.001 | <0.001 | ||
| m-Xylene | Cold meat fat, plastic | MXT-5 | 53.48 (874) | 128.2b | 155.9b | 187.8b | 2,527.7a | 132.1 | <0.001 | <0.001 | <0.001 |
| MXT-1701 | 55.92 (928) | 113.6b | 171.4b | 174.0b | 2,342.4a | 123.9 | <0.001 | <0.001 | <0.001 | ||
| 1,2-diethylbenzene | Fatty, geranium, oily | MXT-5 | 56.89 (899) | 175.7b | 225.8b | 140.3b | 837.0a | 38.4 | <0.001 | <0.001 | <0.001 |
| MXT-1701 | 60.23 (959) | 62.7b | 129.1b | 91.0b | 745.1a | 36.2 | <0.001 | <0.001 | <0.001 | ||






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





