 |
 |
Abstract
The hypochlorite ion (OCl−) is a widely used disinfecting agent in pig rearing in Korea, but its residual effect on CH4 production from pig slurry is unclear. The objective of this study was to investigate the inhibition effects of residual OCl− on CH4 production during the initial anaerobic digestion stage of pig slurry. Three organic concentrations (9.9, 26.2 and 43.7 g/L) of volatile solids (VS) were tested with the addition of 52.3 mg/L OCl−, ten times of the typical concentration used in Korea, or without OCl− (Control) in anaerobic batch culture. The culture was run under mesophilic (38°C) conditions for 20 d. At the lowest organic concentration with OCl−, the VS degradation was 10.3% lower (p<0.05) than Control, while at the higher organic concentration with OCl−, it did not differ from Control. CH4 yields were higher in the control treatments than their OCl− counterpart cultures, and CH4 yields of Control and OCl− treatments at the organic concentrations of 9.9, 26.2 and 43.7 g/L differed in the probability level (p) of 0.31, 0.04, and 0.06, respectively. Additionally, CH4 concentration increased steeply and reached 70.0% within 4 d in the absence OCl−, but a gradual increase up to 60.0% was observed in 6 d in the OCl− treated cultures. The Rm (the maximum specific CH4 production rate) and λ (lag phase time) of 9.9 g/L with OCl− were 8.1 ml/d and 25.6 d, while the Rm was increased to 15.1 ml/d, and λ was reduced to 11.4 d in PS-III (higher organic concentration) with OCl−. The results suggest that a prolonged fermentation time was necessary for the methanogens to overcome the initial OCl− inhibitory effect, and an anaerobic reactor operated with high organic loadings was more advantageous to mitigate the inhibitory effect of residual hypochlorite ion.
The hypochlorite ion (OCl−) is a widely used disinfecting agent to sterilize pig pens and to prevent animal diseases spreading in Korea. The hypochlorite ion is typically sprayed onto the pig pen floor and could be retained in the pig slurry when it is discharged from the pig farmhouse. The residual hypochlorite ion in the collected pig slurry may inhibit the anaerobic microbial activity during the anaerobic fermentation for the biogas production.
Hypochlorite ion is a non-selective, highly reactive oxidant for a wide range of cellular and subcellular compounds, and inactivation of enzymes and interference of DNA synthesis were reported (Albrich et al., 1981; McKenna and Davies, 1988; Rakita et al., 1990; Ducan and Daniele, 1996). But its actual mechanism of action is not fully known (McDonnell and Russell, 1999). No microorganism is known to possess a specific enzymatic mechanism for the detoxification of hypochlorite ion (Leyer and Johnson, 1997). Hypochlorous acid is dissociated as H+ and OCl−, and the pKa of HOCl is 7.53. Metcalf (2002) reported that the protonated form (HOCl) has a more toxic effect than the deprotonated form (OCl−). When hypochlorite ions react with ammonium in aqueous solution, chloramine (NH2Cl) is formed. Shih and Lederberg (1976) reported that NH2Cl induces lesions in DNA and cause a disinfection effect. The non-selective and highly oxidative characteristics of hypochlorite ions result in reactions with various types of organic matter. Thus the disinfecting power of hypochlorite ion could be reduced by oxidation of organic matter other than microorganisms (Metcalf, 2002).
Biogas is generally produced through anaerobic biological processes that convert biodegradable organic materials into methane (CH4) and carbon dioxide (CO2). The biogas production is carried out by the sequential microbial reactions of hydrolysis of biodegradable organic materials (Hydrolysis), fermentation of the hydrolyzed products (Acidogenesis), formation of substrates (acetic acid, CO2, H2) for CH4 fermentation (Acetogenesis), and CH4 production (Methanogenesis). The CH4 producing microbial community consists of different types of bacteria within the sequential mechanism of CH4 production as described above. Therefore, stability of anaerobic microbial communities and balance between microbial populations within the sequential reactions are very important in the operation of an anaerobic digester (Yu et al., 2005).
The objective of this study was to assess the effect of a residual disinfecting agent, hypochlorite ion, on CH4 production in the initial methanogenic anaerobic digestion of pig slurry. In order to investigate the alleviation effect of organic materials present in pig slurry on the disinfecting agent, the effect of hypochlorite ion on CH4 production in different organic concentrations was also analyzed.
The hypochlorite ion disinfecting agent used in this study was Harasol® (Yuhan Co., Korea) consisting of 99.9% sodium hypochlorite (NaOCl) as the active ingredient. Sodium hypochlorite is commonly used for the sanitary management of pig pens and the prevention of animal diseases. The pig slurry discharged from the pig pen was a mixture of feces, urine, and waste water that includes washing water and leaked water from a pig pen. It was collected and stored at the pig slurry storage tank for the anaerobic digestion. The concentration of hypochlorite ion in the discharged pig slurry was approximately 5.23 mg/L based on the recommended rate of Harasol® usage (300 ml/m2 of Harasol®, diluted 200 times), typical pig housing density (0.6 m2/heads) in Korea and the discharging volume (8.6 kg/head/d) of pig slurry (KME, 1999; KMFAFF, 2008). Considering the overuse of disinfecting agent and the direct introduction of the discharged pig slurry to a farm-scale anaerobic digester at the intensive livestock breeding system of Korea, a ten times hypochlorite ion concentration (52.3 mg/L) was used in our study.
The inhibitory effect of hypochlorite ions on the anaerobic biogas production was investigated at 0 (Control) or at 52.3 mg/L OCl− concentration using three different organic concentrations. The three organic concentrations were 9.9, 26.2 and 43.7 g/L volatile solid (VS) contents, representing treatment PS-I, PS-II, and PS-III, respectively. All treatments were replicated three times.
For the anaerobic inhibition assay, pig slurry was obtained from a slurry storage tank in a pig farm. To prepare uniform samples with similar chemical properties, macro-particles were removed from pig slurry samples by stepwise sieve screening (pore diameter, 1 and 2 mm). Prior to initiating the anaerobic inhibition assay, 2 L slurry samples having different organic concentrations were fermented in 5 L anaerobic batch reactors for 3 d under mesophilic (38°C) conditions. When the CH4 concentration in the biogas reached 60.0%, 70 ml of pre-fermented digestate was dispensed to 120 ml size serum bottles for the anaerobic inhibition assay. Either 0 or 0.73 ml of 200 times diluted sodium hypochlorite solution was dispensed to the bottles resulting in the OCl− residual concentration of 0 or 52.3 mg/L. The pre-fermented digestate had a volatile solid (VS) content of 64.0% in the total solid (TS) fraction and a pH value of 7.43 (Table 1).
The head space of serum bottle was filled with N2 gas, and sealed with a butyl rubber stopper. All the treatments in triplicate were incubated for 20 d in the convection incubator of mesophilic temperature (38°C). The bottles were shaken with hand daily during the incubation period. The modified Gompertz equation, employed to fit the cumulative CH4 production data, is shown as follows.
Where M is cumulative CH4 production (ml); e is exp (1); Rm is the maximum specific CH4 production rate (ml/d); P is CH4 production potential (ml); λ is lag phase time (d).
Total solid, volatile solid, pH, soluble chemical oxygen demand (SCOD), total chemical oxygen demand (TCOD), total nitrogen (TN) and ammonium nitrogen (NH4+-N) were determined according to standard methods (APHA, 1998). Total gas production was measured daily for the first 5 d and every 2 to 3 d afterward by displacement of an acidified brine solution in burette and recording the volume of displaced solution after correcting to atmospheric pressure (Beuvink et al., 1992; Williams et al., 1996). To investigate the gas composition, gas samples were collected daily for the first five days and every 2 to 3 d thereafter. The CH4 and CO2 concentration in the gas samples were determined by gas chromatograph (Model GC2010, Shimazhu, Japan) equipped thermal conductivity detector with the HayeSep Q packed column (CRS Inc., Louisville, KY, USA). Column was operated with helium as the carrier gas at a constant flow rate of 5 ml/min. The injector was maintained at 150°C, the oven was set at 90°C, and the detector was set at 150°C. The CH4 yield was calculated by multiplying the total gas volume produced by the CH4 concentration. The difference of two means (with and without OCl−) within same organic concentration was analyzed at p = 0.05 by Student t-test using the SAS (SAS v 9.1; SAS Inst. Inc., Cary, NC, USA).
At the end of 20-d fermentation, hypochlorite ion addition had no effect (p>0.05) on pH, NH4+-N, TN and SCOD concentrations in the anaerobic batch reactor fermented in all three organic concentrations (Table 2). Total solids and VS decreased during the fermentation for inhibition assay in both 0 and 52.3 mg/L OCl− cultural serials. For the Control (0 mg/L OCl−) culture series, the VS contents were reduced to 27.5, 24.4, and 33.1% at the end of 20-d fermentation, compared with the initial VS contents for PS-I, PS-II, and PS-III, respectively. Besides, for the 52.3 mg/L hypochlorite ion treated culture series, the VS contents were decreased to 17.2, 19.3, and 27.3% over a 20 d fermentation period for PS-I, PS-II, and PS-III, respectively. Despite of the significant VS reduction (p<0.05) in OCl− culture of PS-I with VS loading of 9.9 g/L, none of the reductions in TCOD and SCOD were significant (p>0.05). Additionally, inhibition by OCl− was significant at the lowest organic loading (PS-I) as presented by the significant differences (p<0.05) in VS degradability (VS reduction of 17.2% with OCl−, VS reduction of 27.5% without OCl−). For the two higher organic concentrations PS-II and PS-III, the differences in VS degradability between cultures of with and without OCl− were (p>0.05) much smaller than the difference of VS degradability between of cultures with and without OCl− in PS-I.
CH4 production in the Control cultures without OCl− increased exponentially from day zero to six, and continued to increase steadily at a lower rate from day eight to the end of fermentation (d 20). In the assay cultures with OCl−, the slow, but steady increase in CH4 production was observed in the fermentation period from d 0 to 11. Thereafter, the CH4 yield increased more rapidly than that of fermentation period of d 0 to 6 (Figure 1). During anaerobic batch fermentation, CH4 content increased steeply and reached 70.0% within 4 d for the culture series without OCl−. In contrast, CH4 content of culture series with OCl− was below 60.0% during 6 d after fermentation set up, gradually increased above 60.0% from 14th d of fermentation (Figure 2). The total CH4 yields were 0.21, 0.15, and 0.13 L/g-VSadded in the organic concentrations PS-I, PS-II and PS-III without OCl− anaerobic culture series and these values were higher than 0.17 (p = 0.31), 0.13 (p = 0.04), and 0.10 (p = 0.06) L/g-VSadded, respectively, of assay culture series with OCl−, (Table 2). These results meant that CH4 yields (L/g-VSadded) from culture series having higher organic concentration, with or without OCl−, showed lower CH4 yield. These results were attained in the short fermentation period because CH4 production was completed in the reactor with low organic concentration during the fermentation time of 20 d, while CH4 production was ongoing in the reactor with higher organic concentration.
Figure 3 shows cumulative CH4 production and the curve fitted by the modified Gompertz equation in OCl− inhibition assay cultures of pig slurry having different organic concentrations. The maximum specific CH4 production rate (Rm), CH4 production potential (P), and lag phase time (λ) that were estimated by the modified Gompertz equation are shown in Table 3. In PS-I with the lowest organic concentration, PS-I without OCl− gave a P, Rm, and λ that were 140.0 ml, 18.2 ml/d, and 2.2 d, respectively. While the PS-I with OCl- gave a P, Rm, and λ that were 438.5 ml, 8.1 ml/d, and 25.6 d, respectively. This result implies that the presence of OCl− caused a CH4 production rate that was decreased more than two times by the inhibition effect of OCl− in the initial fermentation stage. In this result, the CH4 production potential (438.5 ml) of PS-I with OCl− was higher than that (140.8 ml) of PS-I without OCl−, this was caused by the overestimation of P of PS-I with OCl− due to use of the assay data from an initial short fermentation time (20 d). In PS-III with the highest organic concentration, PS-III without OCl− had a P, Rm, and λ that were 377.1 ml, 40.6 ml/d, and 2.6 d. While P, Rm, and λ of the PS-III with OCl− were 456.0 ml, 15.1 ml/d, and 11.4 d, respectively. Comparing the Rm (8.1 ml/d) and λ (25.6 d) of PS-I with OCl−, the maximum specific CH4 production rate increased to 15.1 ml/d, and the lag phase time was reduced to 11.4 d. These results imply that the higher organic concentration might alleviate the inhibition effect of OCl− by the lessening of lag phase time and increasing the CH4 production rate in the initial stage of fermentation.
Recently regulations and directives on hygiene and sanitary conditions require a high safety level in livestock food products. To meet these requirements, hazard analysis and a critical control points (HACCP) system were introduced to the breeding step at pig farms in Korea. Increasingly high concentrated disinfecting agents were used in order to combat the recent occurrence of various animal diseases. The OCl− ingredient was occasionally used in high concentrations to over 10 times the recommended usage. Our results showed a noticeable inhibitory effect the residual hypochlorite ion on methanogenesis in the initial phase of the 20 d fermentation period. Higher organic loadings in the anaerobic reactor decreased the OCl− inhibitory effect. Our results are inconsistent with those reported by Lambert and Johnston (2001) where hypochlorite ion effectiveness was significantly reduced by organic material through chemical reactions and spatial reaction barriers. Pig slurry has a high amount of colloidal or particulate material as well as soluble organic matter. Those organic materials may compete with bacteria to react with OCl−. The sieving process to remove macro-particles for a uniform initial culture might have diluted the soluble and particulate organic material as well as reducing the anaerobic microorganisms’ population, causing changes in anaerobic microbial activity in our anaerobic fermentation experiment. It is possible that organic materials that bound with microbes in the anaerobic reactors may form the spatial barrier, protecting microbes from the OCl−. Additionally, the effectiveness of disinfectant is also affected by the type of microbes and their growth stages (Maillard, 2002; Kitis, 2004). These effects could have contributed to the differences reported from our study and the one by Lambert and Johnston (2001).
The effectiveness of disinfectants also depends on environmental conditions. Beside microbe population and type, other major factors such as interfering substances, aqueous conditions (pH, temperature), contact time and influence the effectiveness of the disinfectant (Bessems, 1998; Chmielewski and Frank, 2003). Therefore in order to assess the various inhibitory mechanisms of hypochlorite ion on anaerobic fermentation, studies on the operational conditions of the anaerobic reactor, species of anaerobic bacteria, and characteristics of feedstock are needed.
There are limited inhibitory studies on anaerobic microorganisms with most reported on the effect of quaternary ammonium compounds (also a disinfectant) on the methanogenic process (Battersby and Wilosn, 1989; Garcia et al., 1999). Tezel et al. (2006) reported that a prolonged fermentation time was necessary for the methanogens to overcome the initial inhibitory the effect of quaternary ammonium compounds. It seems that the inhibitory effect is reduced by the adsorption of quaternary ammonium compounds on organic material since quaternary ammonium compounds are not biodegraded under anaerobic conditions (Tezel et al., 2006). Our OCl− results are consistent with those of Tezel et al. (2006) with quaternary ammonium compounds since CH4 production was initially depressed and a longer time was needed (11th and 14th d) to reached the maximum CH4 concentration during our OCl− inhibitory fermentation study. Our results suggest that CH4 production cultured under 52.3 mg/L OCl− was stabilized after 11 to 14 d in the batch fermentation. Although our results were obtained from the anaerobic batch culture, a commercial anaerobic digester continuously operated under a high organic concentration might have a higher buffering capacity against the variable inhibitory effects by hypochlorite ion.
During the 20-d anaerobic batch culture assay under mesophilic (38°C) conditions, the residual hypochlorite ion at concentration of 52.3 mg/L in pig slurry showed tan inhibitory effect on CH4 yield and biodegradation of biomass. The inhibitory effect by residual hypochlorite ion could be modified by the chemical reactions with various organic materials during the anaerobic fermentation of pig slurry. CH4 production was preferentially stabilized in the inhibitory assay culture containing a high organic material content. Our results suggested that a prolonged fermentation time was necessary for the methanogens to overcome the initial inhibitory effect. Moreover, an anaerobic reactor operated in high organic loadings is more beneficial in mitigating the hypochlorite ion inhibitory impact.
ACKNOWLEDGMENTS
This work was supported by the New and Renewable Energy Technology Development of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government Ministry of Knowledge Economy (No. 20103030090010).
Figure 1
The inhibition assay curve of hypochlorite ion in anaerobic batch reactors (aPS-I: pig slurry containing VS of 9.9 g/L, PS-II: pig slurry containing VS of 26.2 g/L; PS-III: pig slurry containing VS of 43.7 g/L. b With hypochlorite ion of 52.3 mg/L).

Figure 2
Effect of hypochlorite ion on CH4 concentration in anaerobic batch reactors (aPS-I: pig slurry containing VS of 9.9 g/L; PS-II: pig slurry containing VS of 26.2 g/L; PS-III: pig slurry containing VS of 43.7 g/L. b With hypochlorite ion of 52.3 mg/L).
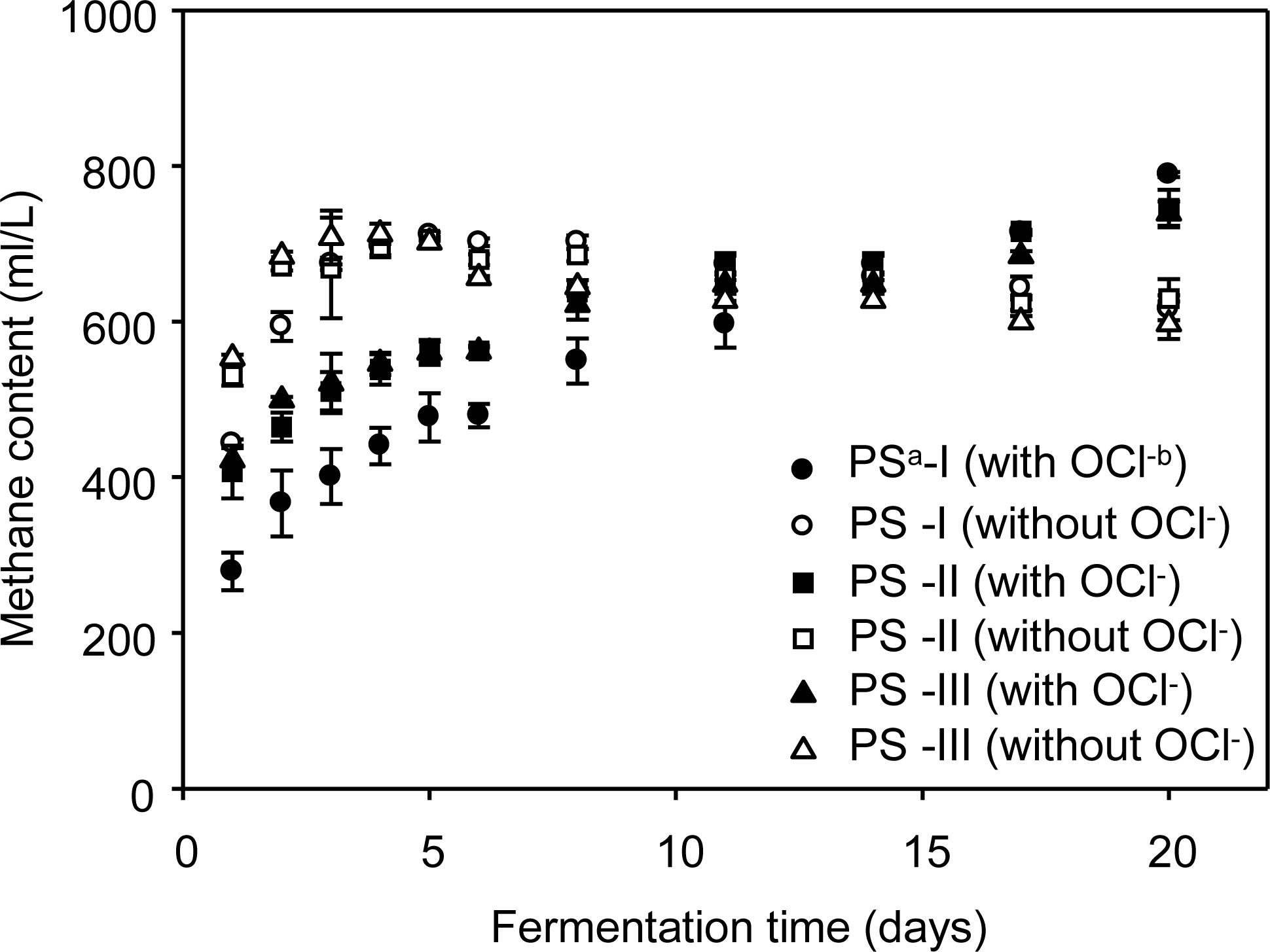
Figure 3
Effect of hypochlorite ion on cumulative CH4 production in anaerobic batch reactors (aPS-I: pig slurry containing VS of 9.9 g/L; PS-II: pig slurry containing VS of 26.2 g/L; PS-III: pig slurry containing VS of 43.7 g/L. b With hypochlorite ion of 52.3 mg/L).
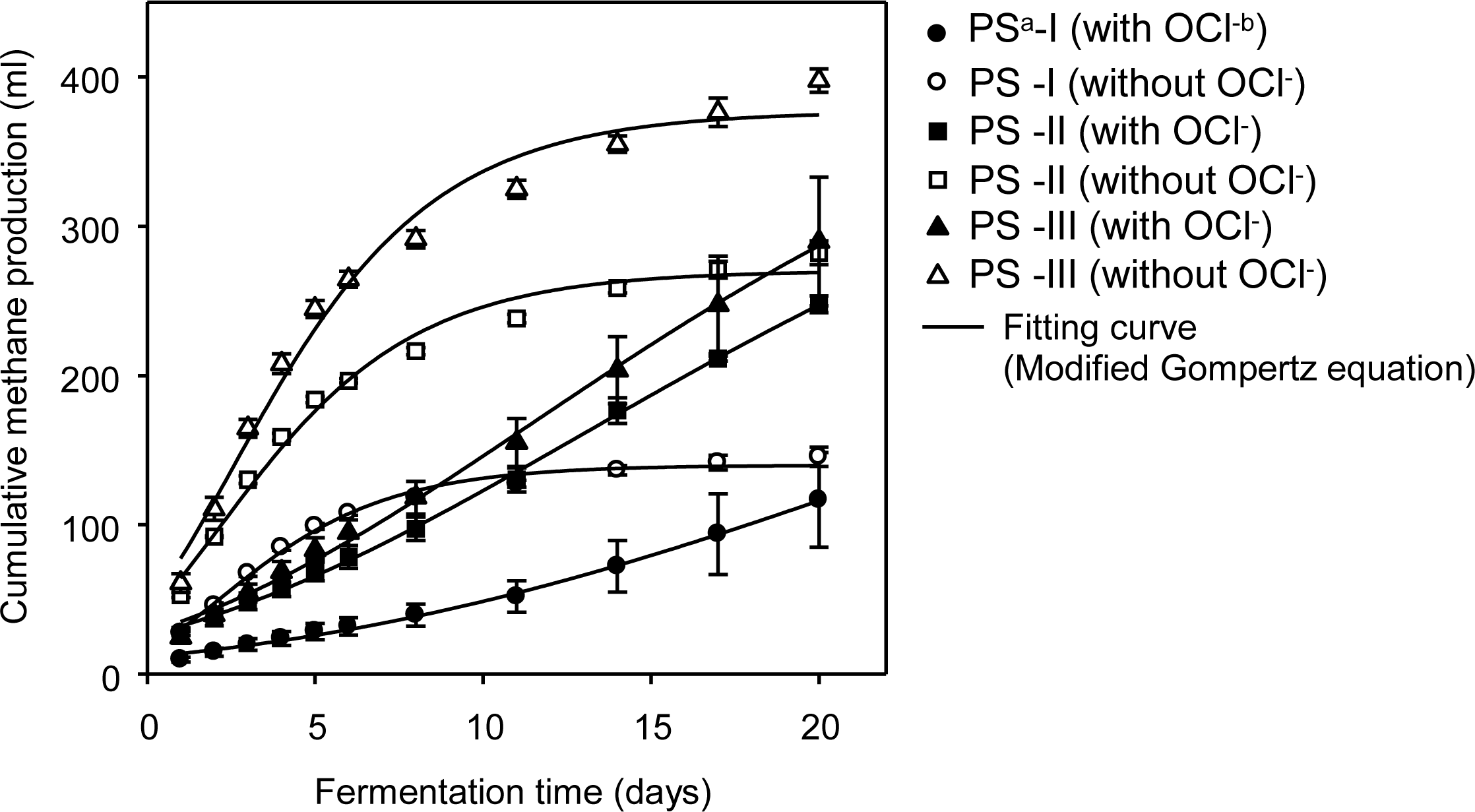
Table 1
Initial chemical composition of the pig slurries (PS) used in the inhibition assay in anaerobic batch reactors
| Item |
Treatment1
|
||
|---|---|---|---|
| PS-I | PS-II | PS-III | |
| TS (g/L) | 15.6±0.52 | 41.3±0.1 | 67.5±0.3 |
| VS (g/L) | 9.9±0.4 | 26.2±0.1 | 43.7±0.1 |
| pH | 7.43±0.02 | 7.43±0.02 | 7.43±0.02 |
| SCOD (g/L) | 1.9±0.1 | 3.4±0.2 | 5.7±0.3 |
| TCOD (g/L) | 18.1±1.2 | 42.4±1.9 | 76.4±3.8 |
| TN (g/L) | 3.2±0.1 | 4.0±0.2 | 4.7±0.1 |
| NH4+-N(g/L) | 1.4±0.02 | 2.4±0.01 | 1.3±0.06 |
Table 2
Final chemical composition of digestates and CH4 yield after the 20-d inhibition assay in an anaerobic batch reactor
| Items |
Treatment1
|
|||||
|---|---|---|---|---|---|---|
|
PS-I
|
PS-II
|
PS-III
|
||||
|
OCl−
|
OCl−
|
OCl−
|
||||
| Without | With2 | Without | With | Without | With | |
| TS (g/L) | 12.9a3±1.04 | 13.7b±0.3 | 33.0a±0.6 | 34.2a±0.5 | 51.2a±0.5 | 55.4a±0.6 |
| VS (g/L) | 7.2a±0.7 | 8.2b±0.3 | 19.8a±3.2 | 21.2a±1.8 | 29.2a±1.5 | 31.8a±3.0 |
| pH | 7.49±0.03 | 7.46±0.03 | 7.39±0.02 | 7.37±0.02 | 7.33±0.06 | 7.31±0.02 |
| SCOD (g/L) | 2.4±0.08 | 2.0±0.01 | 2.9±0.04 | 3.0±0.03 | 3.4±0.10 | 3.6±0.03 |
| TCOD (g/L) | 14.9±3.0 | 15.7±0.4 | 31.5±3.9 | 30.7±1.1 | 27.1±4.3 | 48.8±4.0 |
| TN (g/L) | 1.9±0.1 | 3.1±0.04 | 3.5±0.2 | 3.3±0.2 | 4.2±0.2 | 4.4±0.1 |
| NH4+-N (g/L) | 1.6±0.06 | 1.6±0.11 | 2.6±0.02 | 2.5±0.06 | 3.1±0.13 | 3.1±0.05 |
| VS removal (%) | 27.5a±3.3 | 17.2b±1.2 | 24.4a±5.9 | 19.3a±5.3 | 33.1a±5.7 | 27.3a±2.9 |
| CH4 yield (L/g-VSadded) | 0.21a±0.01 | 0.17a±0.03 | 0.15a±0.002 | 0.13b±0.002 | 0.13a±0.002 | 0.10a±0.01 |
Table 3
Analysis of cumulative CH4 production by modified Gompertz equation in 20-d OCl− inhibition assay curve
| Treatment |
P2 ml |
Rm3 ml/d |
λ4 Days |
||
|---|---|---|---|---|---|
| PS-I1 | OCl− | Without | 140.0 | 18.2 | 2.2 |
| With5 | 438.5 | 8.1 | 25.6 | ||
| PS-II | OCl− | Without | 270.2 | 30.1 | 2.2 |
| With | 436.4 | 12.9 | 12.9 | ||
| PS-III | OCl− | Without | 377.1 | 40.6 | 2.6 |
| With | 456.0 | 15.1 | 11.4 | ||
REFERENCES
Albrich JM, McCarthy CA, Hurst JK. 1981. Biological reactivity of hypochlorous acid: Implications for microbiocidal mechanisms of leukocyte myelperoxidase. Proc Natl Acad Sci USA 78:210–214.



APHA. 1998. Standard methods for the examination of water and wastewater. 20th ednAmerican Public Health Association; Washington, DC, USA:
Battersby NS, Wilson V. 1989. Survey of the anaerobic biodegradation potential of organic chemicals in digesting sludge. Appl Environ Microbiol 55:433–439.



Bessems E. 1998. The effect of practical conditions on the efficiency of disinfectants. Int Biodeterior Biodegradation 41:177–183.

Beuvink JM, Spoelstra SF, Hogendrop RJ. 1992. An automated method for measuring the time course of gas production of feedstuffs incubated with buffered rumen fluid. Netherlands J Agric Sci 40:401–407.

Chmielewski RAN, Frank JF. 2003. Biofilm formation and control in food processing facilities. Compr Rev Food Sci Food Saf 2:22–32.


Ducan S, Daniele T. 1996. Hypochlorous acid stress in Escherichia coli: Rresistance, DNA damage, and comparison with hydrogen peroxide stress. J Bacteriol 178:6145–6150.



Garcia MT, Campos E, Sanchez-Leal J, Ribosa I. 1999. Effect of the alkyl chain length on the anaerobic biodegradability and toxicity of quaternary ammonium based surfactants. Chemosphere 38:3473–3483.


KME. 1999. The discharging unit of livestock wastes. Notification number 1999-109.Korean Ministry of Environment; Seoul, Korea:
KMFAFF. 2008. The criteria concerned with livestock breeding density. Notification number 2008-79.Korean Ministry of Food, Agriculture, Forestry and Fisheries; Seoul, Korea:
Lambert RJW, Johnston MD. 2001. The effect of interfering substances on the disinfection process: a mathematic model. J Appl Microbiol 91:548–555.


Leyer GL, Johnson AE. 1997. Acid adaptation sensitizes Salmonella typhimurium to hypochlorous acid. Appl Environ Microbiol 63:461–467.



McDonnell G, Russell AD. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 12:147–179.



McKenna SM, Davies KJA. 1988. Bacterial killing by phagocytes: Potential role(s) of hypochlorous acid and hydrogen peroxide in protein turnover, DNA synthesis, and RNA synthesis. Basic Life Sci 49:829–832.


Metcalf E. 2002. Wastewater Engineering: Treatment and Reuse. 4thh ednMcGraw-Hill; New York, USA:
Rakita RM, Michel BR, Rosen H. 1990. Differential inactivation of Escherichia coli membrane dehydrogenases by a myeloperoxidase-mediated antimicrobial system. Biochemstery 29:1075–1080.

Shih KL, Lederberg J. 1976. Effects of chloramine on Bacillus subtilis deoxyribonucleic acid. J Bacteriol 125:934–945.



Tezel U, Pierson JA, Pavlostathis SG. 2006. Fate and effect of quaternary ammonium compounds on a mixed methanogenic culture. Water Res 40:3660–3668.


Williams A, Amat-Marco M, Collins MD. 1996. Pylogenetic analysis of Butyrivibrio strains reveals three distinct groups of species within the Clostridium subphylum of gram-positive bacteria. Int J Syst Evol Microbiol 46:195–199.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





